Case of the Week #641
(1) Hung Vuong hospital; (2) UCSF Health, San Francisco, California, USA
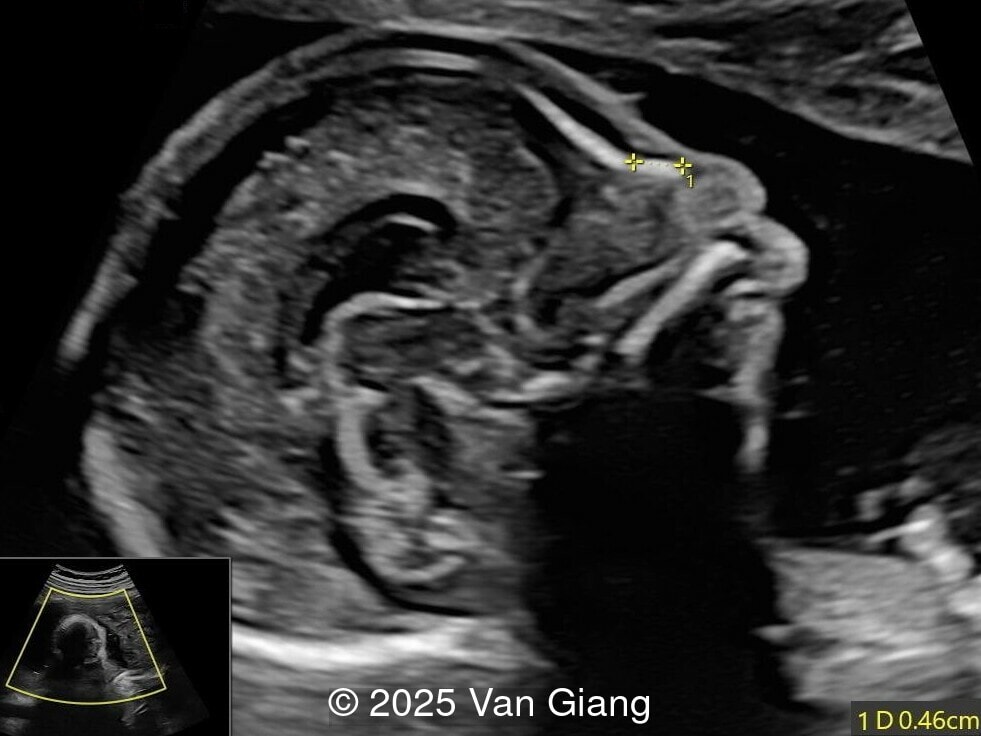
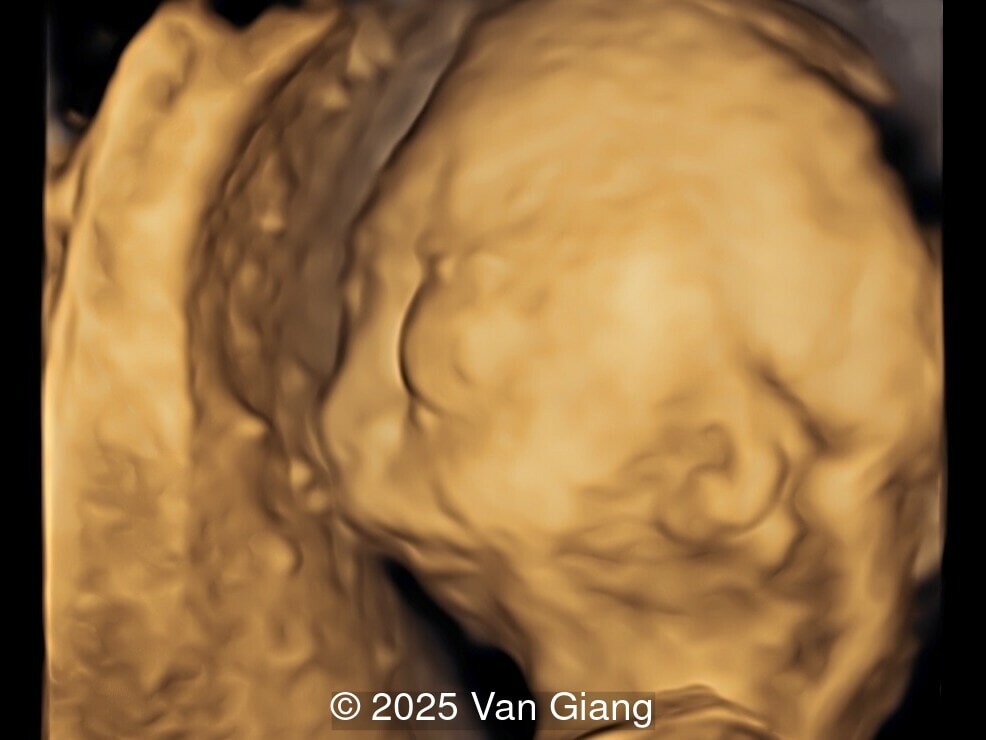
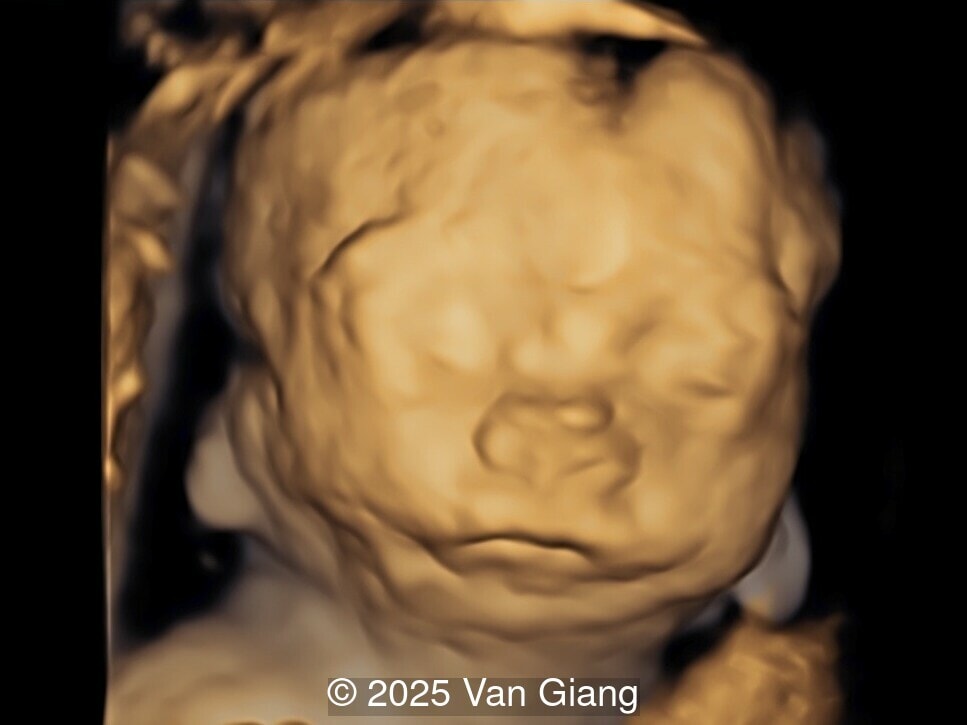
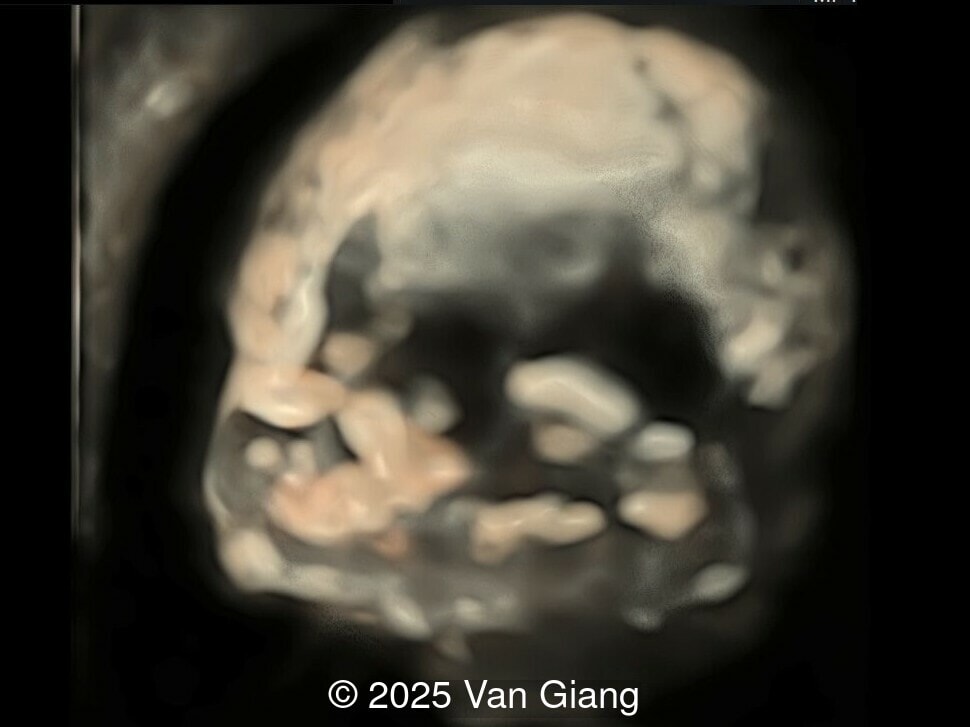
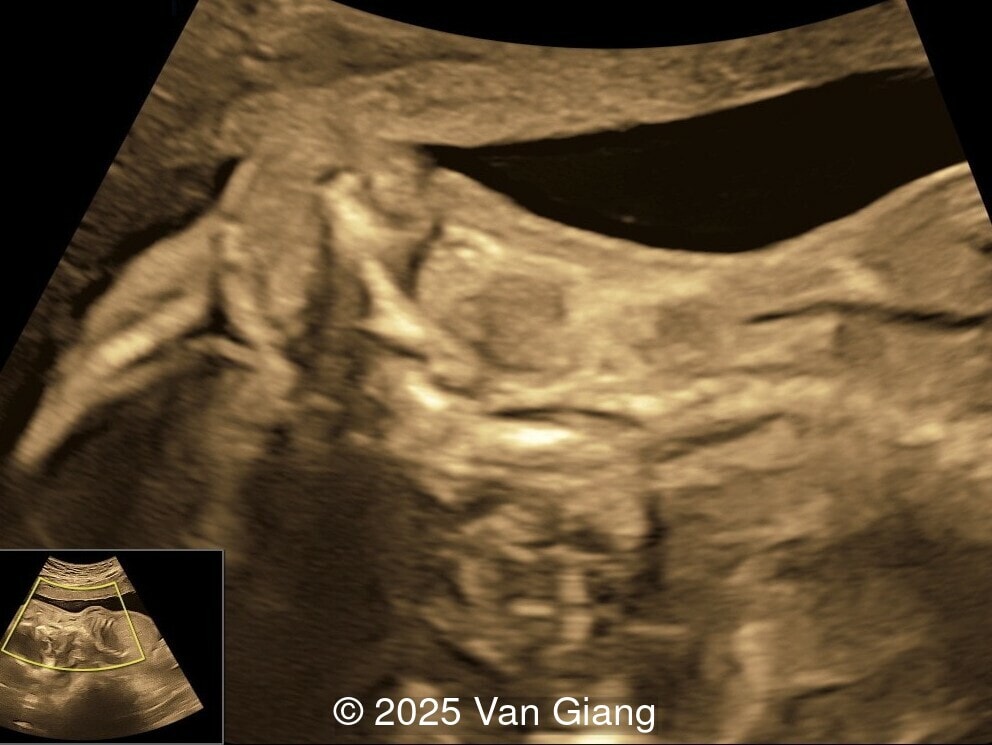
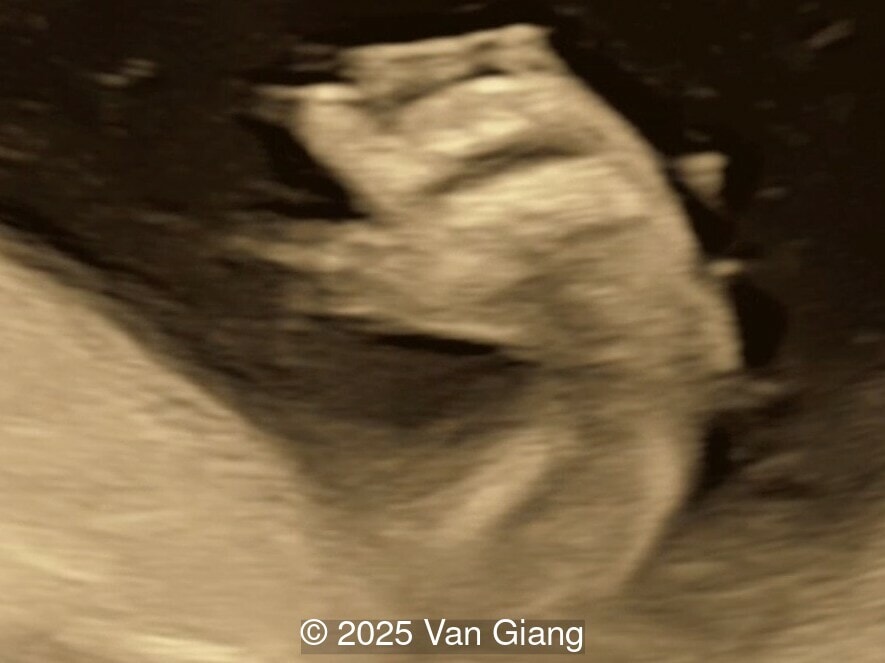
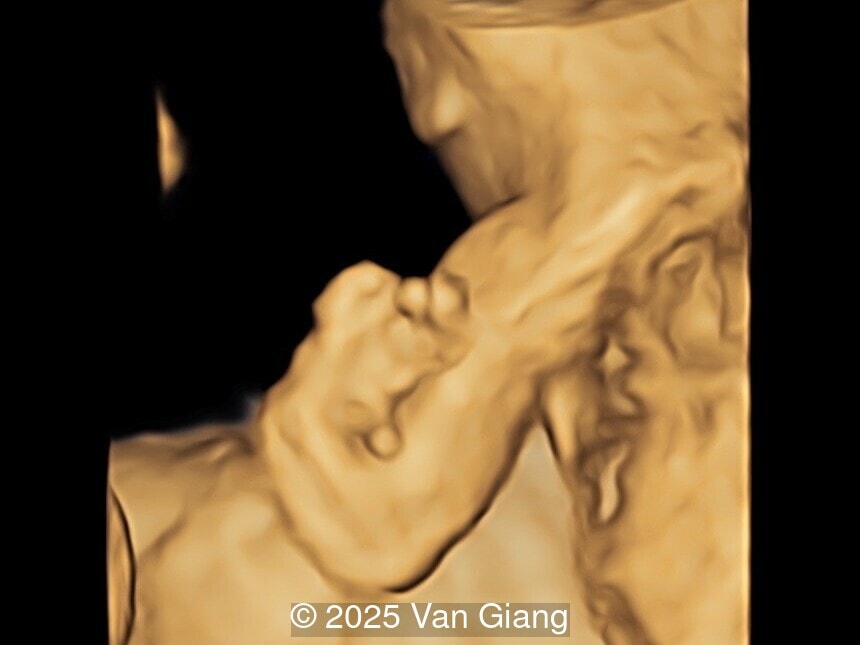
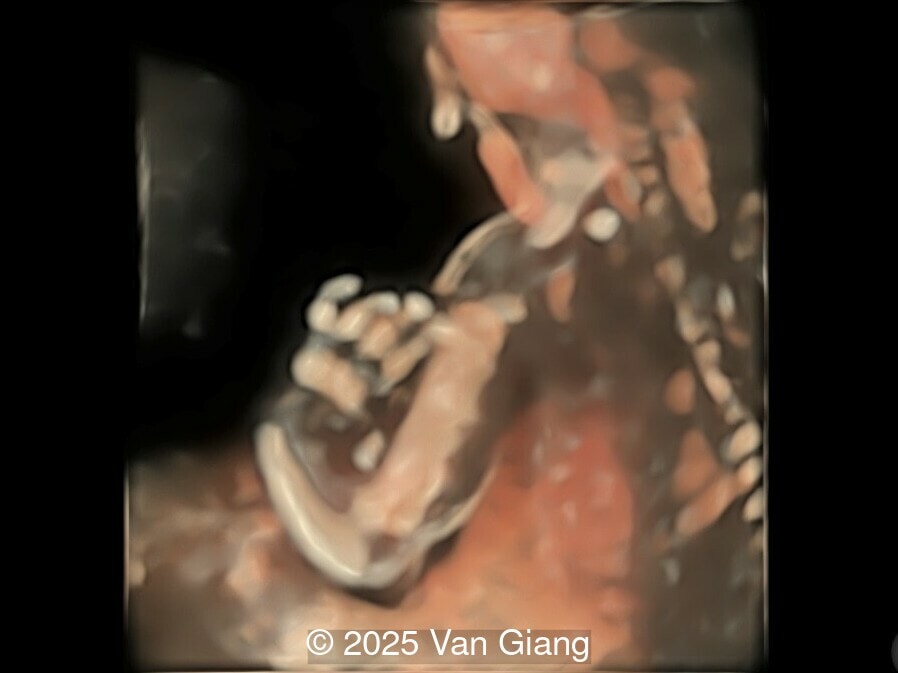
A 32-year-old primigravida presents at 20 weeks gestation without first trimester screening. Ultrasound reveals a small for gestational age fetus with the following anomalies:
View the Answer Hide the Answer
Answer
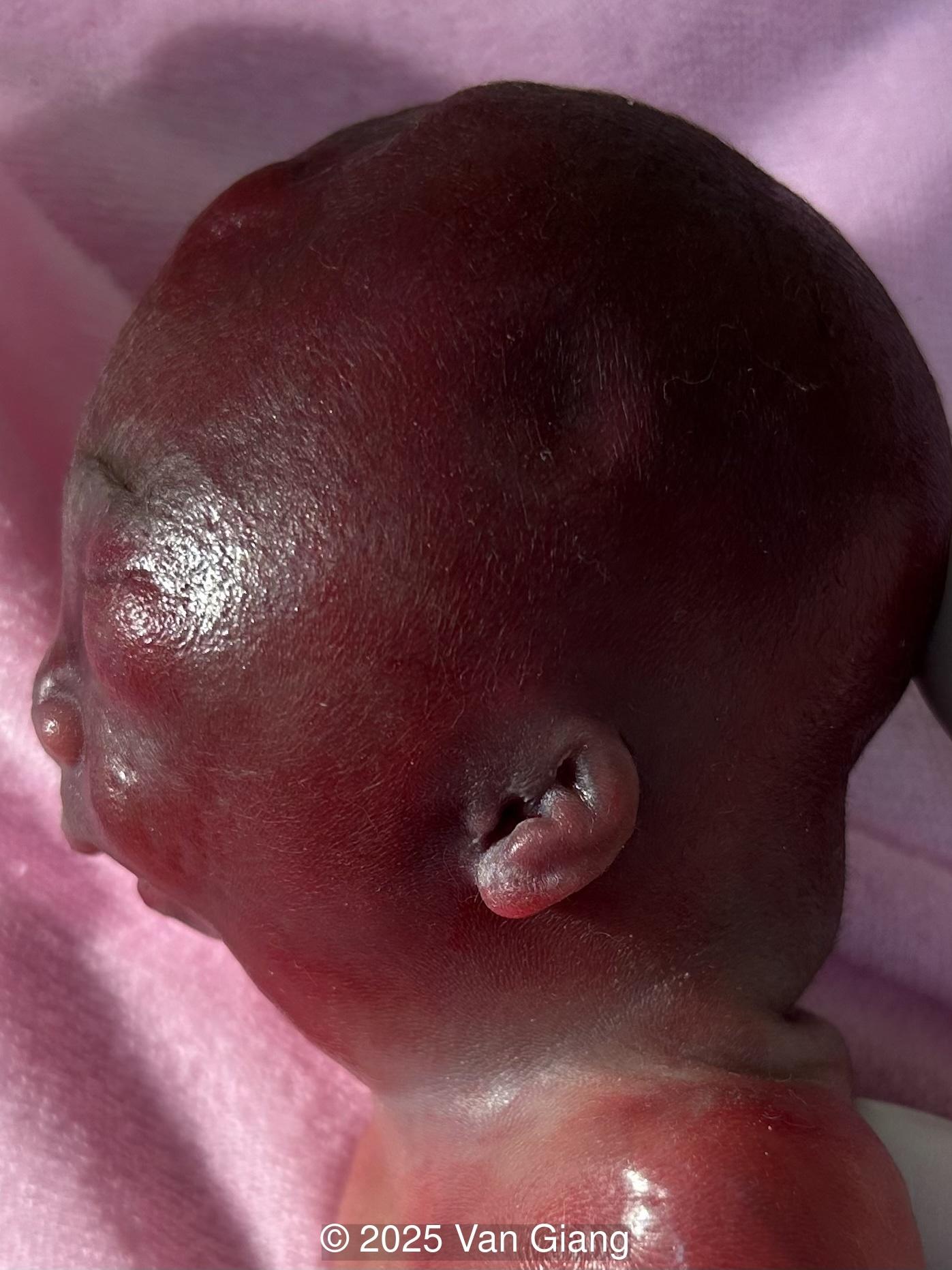
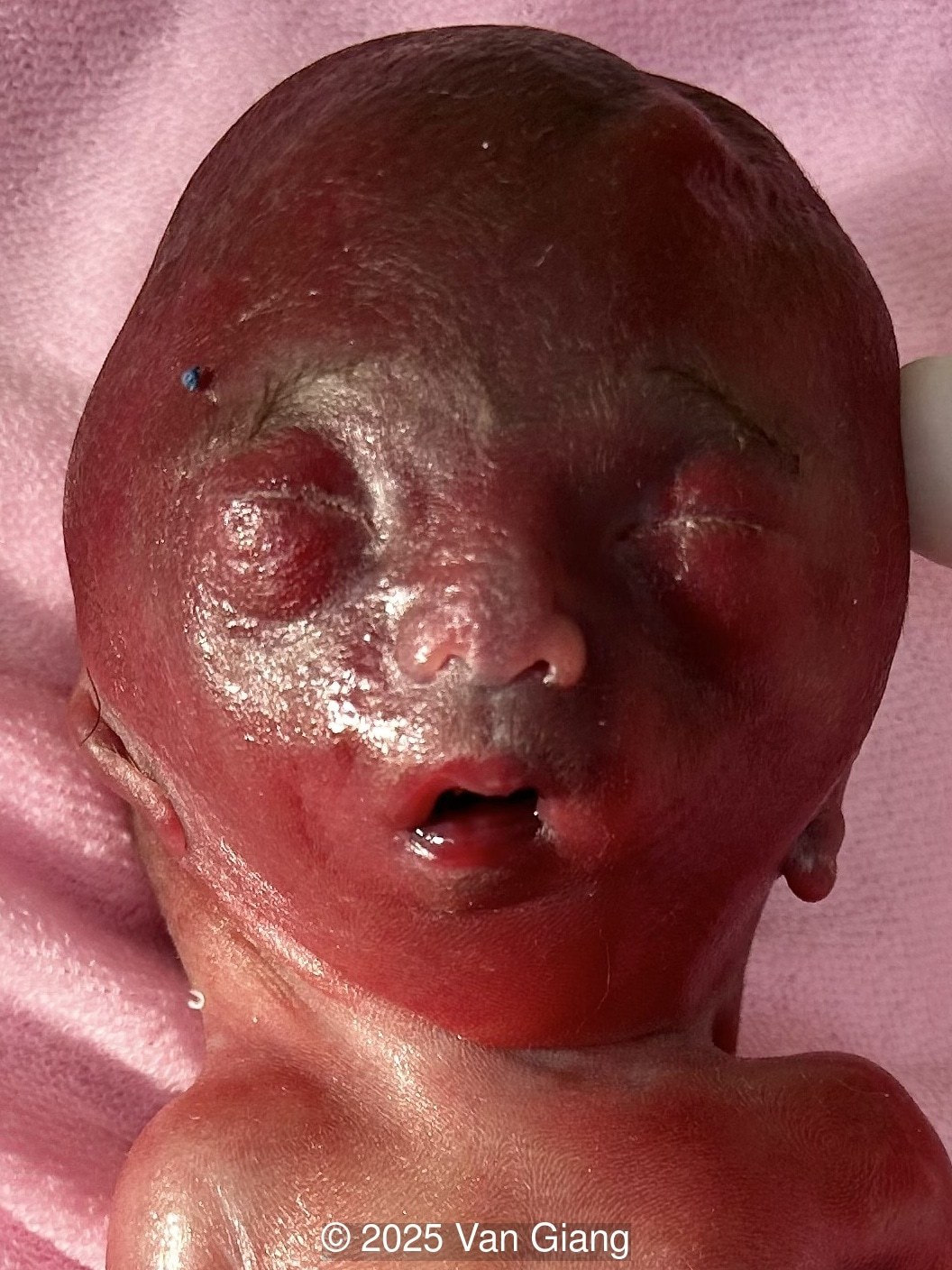
We present a case of Nager syndrome.
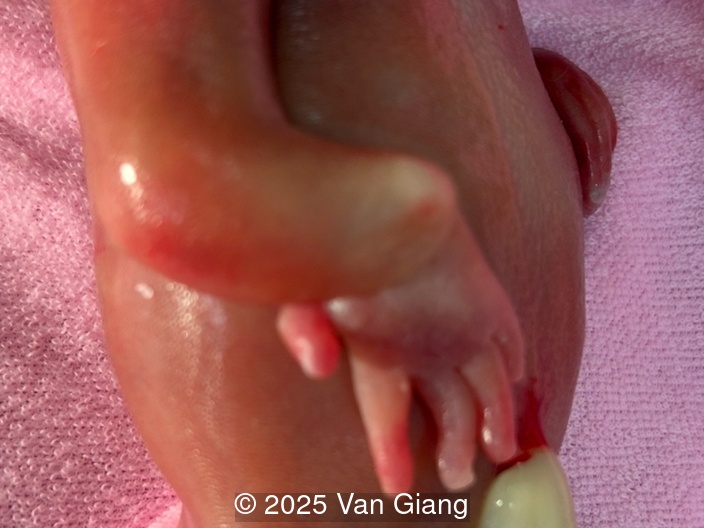
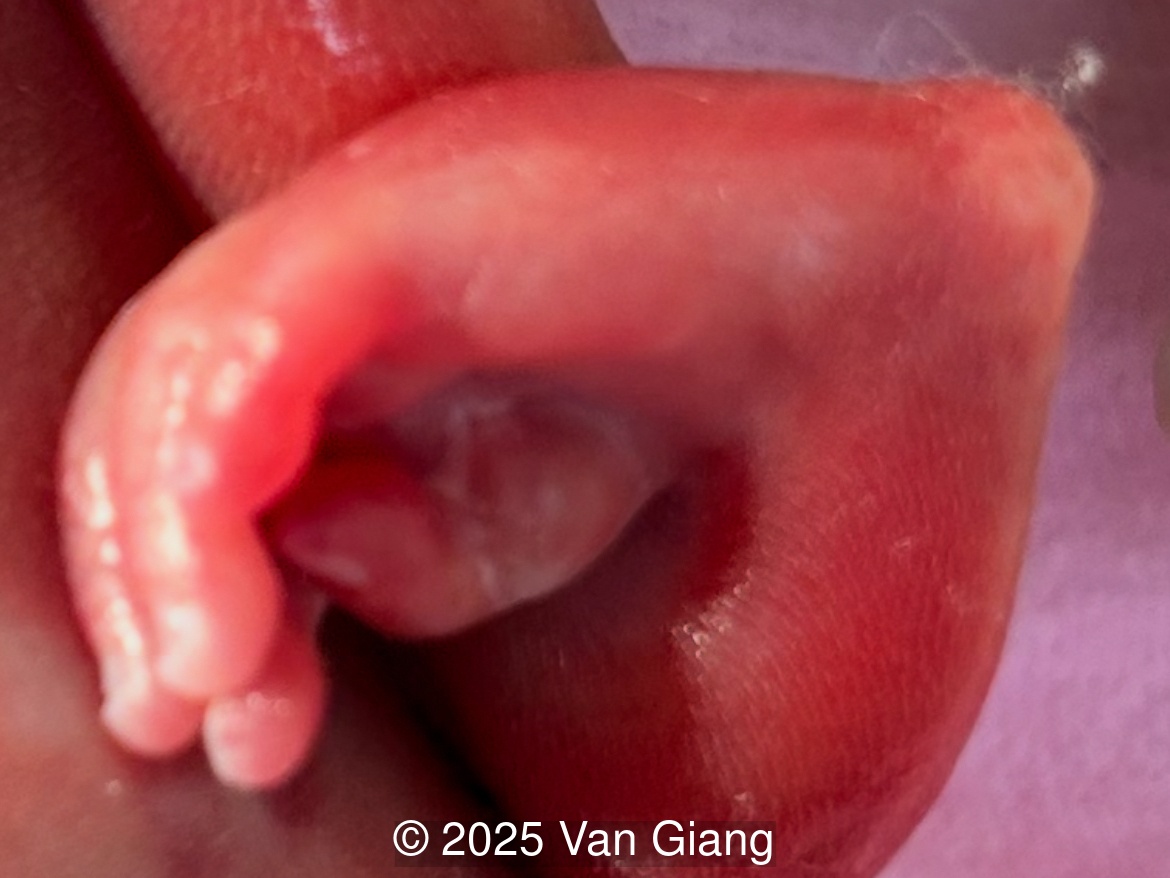
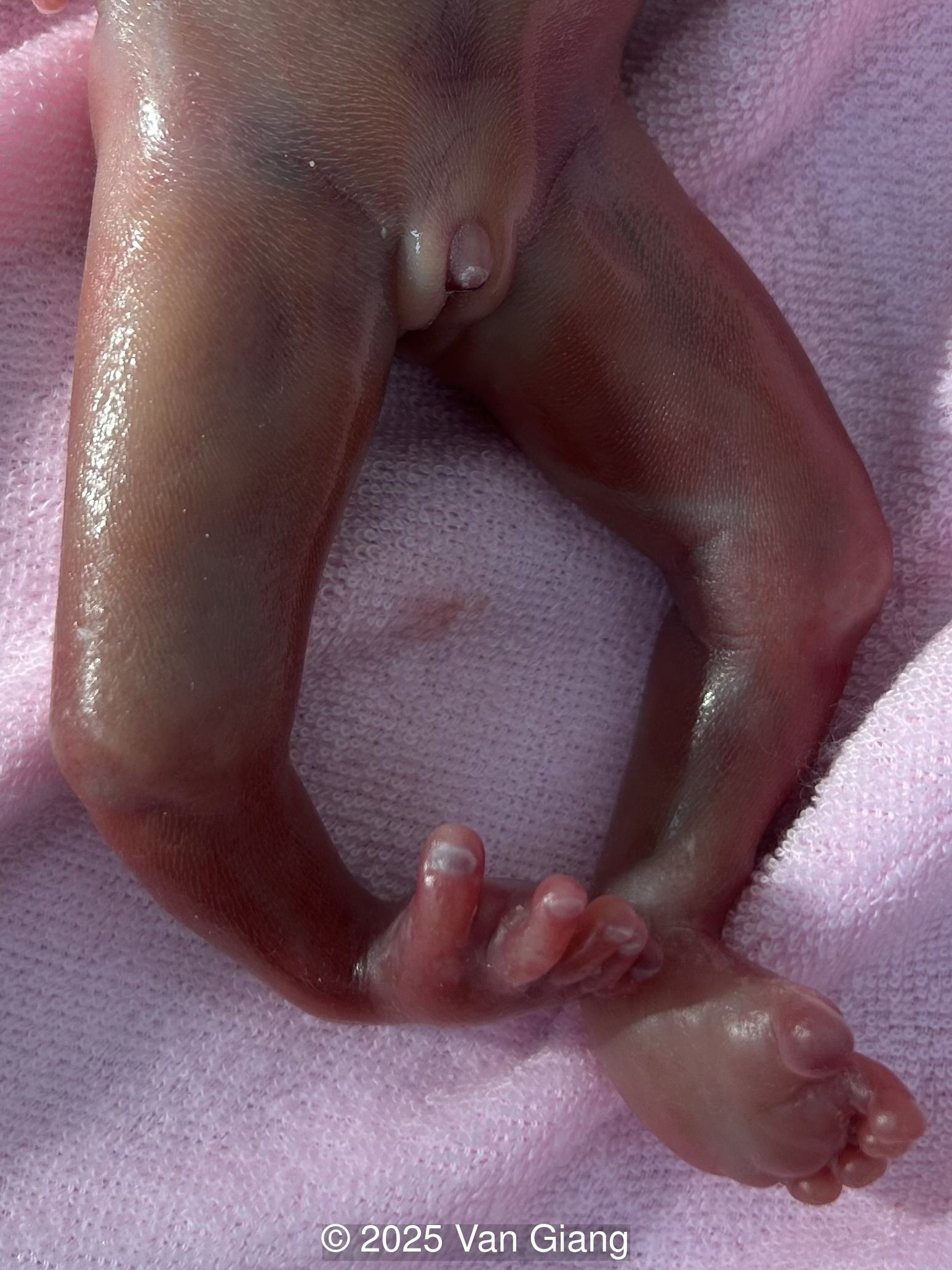
Ultrasound revealed severe mandibular and zygomatic hypoplasia, glossoptosis, cleft palate, and micrognathia with an abnormal facial profile. Both upper limbs showed radial ray sequence with abnormal thumbs and shortened forearms. Unilateral clubfoot was also noted. No major cardiac or visceral anomalies were observed. Based on these findings, Nager syndrome was suspected.
After observing multiple anomalies, the patient elected to terminate the pregnancy and did not consent for genetic testing.
Discussion
Nager syndrome, first described by Nager and De Reynier in 1948 [1], is a rare acrofacial dysostosis characterized by malformations of the craniofacial skeleton and limbs. Patients present with micrognathia, malar hypoplasia, downslanting palpebral fissures, external ear abnormalities with or without external auditory canal atresia, cleft palate, and shortened or absent radii and thumbs [2]. Limb findings are often not symmetric, but both upper limbs are usually involved. Severity can range from mild hypoplasia of the thumb to phocomelia. Rarely, unilateral limb findings are present [3]. While not typical, lower limb anomalies and cardiac defects can be seen [4]. In 2012, Bernier et al identified SF3B4 as the causative gene in Nager syndrome [5]. The SF3B4 gene is located on chromosome 1q21.2 and encodes SAP49, a spliceosomal protein which forms mature messenger RNA involved in limb and craniofacial development [5-7]. Approximately 58-76% of patients with Nager phenotype have mutations in SF3B4 [4-7].
Rodgriguez syndrome was initially reported as a distinct condition [8], though it is now considered a more severe form of Nager syndrome with phocomelia, lower extremity involvement, arhinencephaly, congenital heart defects, and abnormal lung segmentation [9-10]. Skeletal defects that are more commonly found in Rodriguez syndrome are radiohumeral synostosis, absent ulna, hypoplastic scapulae, absent/hypoplastic fibula or tibia, and hypoplastic pelvic girdle [10]. Patients with Rodriguez phenotype also have mutations in SF3B4 [10-11].
In reviewing the literature, we found 68 articles on Nager and Rodriguez syndromes in the English language from 2000 until 2025 that reported on 210 cases [3-6,9-10,12-77], of which 12 were described as Rodriguez phenotype [38-45]. Drivas et al previously summarized the characteristics of patients with Rodriguez syndrome [10]. Regarding patients described as having Nager syndrome, 35 articles provided clinical characteristics on 106 patients [3-6,46-77]. Facial characteristics of Nager syndrome include micrognathia (93%), abnormal ears (79%), downslanting palpebral fissures (63%), and orofacial cleft (40%). Regarding the upper extremities, 84% report an abnormal or absent thumb, and 67% report shortened and abnormal upper extremities, which involves the radius in 52% and radioulnar synostosis in 44%. While limb defects in Nager syndrome are described as pre-axial, the 5th finger is abnormal in 5% of cases and the ulna in 13% of cases. The lower limb is affected in 16%, which is most often clubfoot, though the fibula is shortened or absent in 5%. Other anomalies occur in 30% of patients with Nager syndrome and include cardiac (14%), genitourinary (8%), skeletal (7%), and congenital diaphragmatic hernia (4%).
The first prenatally diagnosed case of Nager syndrome was reported in 1988 by Benson et al [2]. Since that time 24 cases have been prenatally diagnosed in the literature (Table 1) [2-4,9,42-45,67-78]. Characteristic features on prenatal ultrasound include micrognathia, abnormal external ear, upper limb reduction defects, and polyhydramnios. Rarely, organ anomalies are identified such as cardiac defects [45,77-78] and diaphragmatic hernia [72].
Study | GA | Face | Limb | Other | Genetics | Outcome |
|---|---|---|---|---|---|---|
Prenatal findings are listed. If new findings are identified on postnatal exam or autopsy, they are listed in postnatal findings.*marks cases diagnosed as Rodriguez syndrome. ^Monochorionic-diamniotic twin pregnancy with both twins affected. GA: gestational age in weeks; m: months; TOP: termination of pregnancy; VSD: ventricular septal defect. | ||||||
Benson 1988 | 30 | Prenatal: micrognathia, abnormal ears Postnatal: downslanting palpebral fissures, cleft palate | Prenatal: both upper extremities were truncated with abnormal hands, 4 digits on right hand Postnatal: absence of left fibula | Prenatal: polyhydramnios | Normal karyotype | Alive |
Waggoner 1999 | NA | Prenatal: micrognathia Postnatal: abnormal ears, downslanting palpebral fissures, cleft palate | Prenatal: bilateral radial defects, clubfeet Postnatal: absence of radii, hypoplastic ulna, 4 digits on both hands | Prenatal: cardiac defects Postnatal: dysplastic aortic valve | Proximal deletion of chromosome 1 | Death 2m |
Wessels 2002* | 25 | Prenatal: micrognathia Postnatal: microtia, cleft palate | Prenatal: upper limb phocomelia, left thumb agenesis, absent fibula | Postnatal: arrhinencephaly, abnormal lung segmentation, 11 ribs | SF3B4 mutation | TOP |
Paladini 2003 | 22 | Prenatal: micrognathia, abnormal ears | Prenatal: forearm shortening with involvement of radius and ulna, right thumb agenesis | None | Normal karyotype | TOP |
Martinez 2004 | 22 | Prenatal: micrognathia | Prenatal: shortening of forearms bilaterally, absence of radius and thumb | None | NA | NA |
Sermer 2007* | 22 | Prenatal: micrognathia Postnatal: low-set ears | Prenatal: humeri short bilaterally, absent radius and ulna, absent fibula, clubfoot | Postnatal: 11 ribs | SF3B4 mutation | TOP |
Verrotti 2007 | 18 | Prenatal: micrognathia, cleft lip and palate Postnatal: low-set ears | Prenatal: phocomelia of upper extremities, bilateral thumb agenesis, absence of both fibula and tibia Postnatal: hypoplastic ileum and pubic bones, hypoplastic bones of both 5th digits | None | Normal karyotype | TOP |
Couyoumjian 2008 | 20 | Prenatal: micrognathia, abnormal ears Postnatal: downslanting palpebral fissures | Prenatal: short left humerus, radius and ulna Postnatal: hypoplastic left thumb | Prenatal: single umbilical artery Postnatal: hypoplasia of the first left rib | NA | TOP |
Ansart-Franquet 2009^ | 22 | Prenatal: microretrognathia Postnatal: cleft palate, low-set ears | Prenatal: shortened radial and ulnar lengths, thumb agenesis Postnatal: radioulnar synostosis | SF3B4 mutation | TOP | |
Rios 2012 | 33 | Prenatal: micrognathia, low-set ears Postnatal: downslanting palpebral fissures | Prenatal: shortening of upper extremity long bones, left thumb agenesis, shortening of right tibia | Prenatal: polyhydramnios, brachycephaly | NA | Neonatal death |
Gana 2013 | 20 | Prenatal: micrognathia, low-set ears Postanatal: downslanting palpebral fissures | Prenatal: bilaterally short forearms, clubfeet Postnatal: left thumb agenesis, hypoplastic right thumb | None | Normal karyotype | Intrauterine death |
Gana 2013 | 20 | Prenatal: micrognathia Postnatal: low-set ears, downslanting palpebral fissures | Prenatal: bilaterally short humeri and agenesis of radius and ulna, clubfeet Postnatal: agenesis of thumbs, short fibula | Postnatal: horseshoe kidney | Normal karyotype | TOP |
Petit 2013 | 28 | Prenatal: micrognathia, downslanting palpebral fissures, abnormal ears | Prenatal: absent thumbs, large and short halluces | None | SF3B4 mutation | NA |
Castori 2014 | 21 | Prenatal: micrognathia Postnatal: downslanting palpebral fissures, low-set ears | Prenatal: shortening upper extremities involving the radial ray Postnatal: aplasia of radius bilaterally and ulna on the right, thumbs agenesis bilaterally | Prenatal: diaphrgmatic hernia | SF3B4 mutation | TOP |
McPherson 2014 | 22 | Prenatal: micrognathia Postnatal: microtia, downslanting palpebral fissures, lower eyelid colobomas | Prenatal: short forearms, absent fibula, clubbed feet Postnatal: absent thumbs, hypoplastic 5th fingers, absence of radius and ulna, hypoplastic 5th toes, absent fibula | Prenatal: polyhydramnios Postnatal: 11 ribs | SF3B4 mutation | Alive |
Lund 2016 | 11 | Prenatal: micrognathia | Prenatal: short long bones, bilateral clubfeet | Prenatal: nuchal translucencey | SF3B4 mutation | TOP |
Marques 2016* | 29 | Prenatal: micrognathia Postnatal: microtia | Prenatal: shortened and malformed appendicular bones, oligodactyly, preaxial polydactyly Postnatal: humeroradial synostosis, absent ulna, malformed radi, absent fibula | Prenatal: microcephaly, coarctation of aorta, polyhydramnios Postnatal: 11 ribs | SF3B4 mutation | Neonatal death |
Marques 2016*^ | 21 | Prenatal: micrognathia Postnatal: microtia | Prenatal: shortened appendicular bones Postnatal: clubfoot, hypoplasia of radii and thumbs, oligodacytly | Prenatal: microcephaly Postnatal: 11 ribs | SF3B4 mutation | TOP |
Hayata 2019 | 26 | Prenatal: micrognathia, down-slanting palpebral fissures Postnatal: low-set ears | Prenatal: bone defects in both forearms, only 4 fingers Postnatal: clubfoot, bilateral radius and ulna defect | Prenatal: polyhydramnios, VSD | SF3B4 mutation | Alive |
Drozniewska 2020 | 13 | Prenatal: micrognathia, cleft lip | Prenatal: abnormal extension and shortened legs bilaterally, bilateral clubfoot | Prenatal: strawberry-shaped skull, increased nuchal translucency | SF3B4 mutation | TOP |
Drendel 2021 | 23 | Prenatal: micrognathia, abnormal ears | Prenatal: left thumb agenesis | None | SF3B4 mutation | NA |
Veduta 2021 | 20 | Prenatal: micrognathia, low-set ears Postnatal: downslanting palpebral fissures | Prenatal: absent radius on the left and shortened radius on the right, clubfoot | Prenatal: ventriculomegaly | Normal karyotype | TOP |
The differential diagnosis includes other acrofacial dysostosis such as Guion-Almeida type and Miller type, in addition to Roberts syndrome and Mohr syndrome [Table 2]. In patients with Guion-Almeida type acrofacial dysostosis, limb anomalies are less common while microcephaly, cardiac defects and esophageal atresia are more common [79]. Miller type acrofacial dysostosis is more likely to present with foot anomalies as well as post-axial limb anomalies including absence or hypoplasia of the 5th digit [80]. In Roberts syndrome, the limb anomalies are generally more severe with 80% of patients affected with phocomelia in all four extremities. Additionally, these patients have growth restriction and microcephaly [81]. Mohr syndrome less commonly presents with micrognathia, and instead presents with orofacial cleft and a lobulated tongue due to hamartomas. Limb anomalies more commonly include bifid thumb / hallux or polysyndactyly of both the hands and feet [82]. Trisomy 18 may rarely present with micrognathia and shortened or absent long bones, however there is often growth restriction and organ system anomalies including congenital heart disease, omphalocele, and central nervous system defects [84]. Other conditions such as Treacher-Collins [85] and Goldenhar syndrome [86] may present with micrognathia, but do not typically have limb anomalies. Holt-Oram syndrome presents with radial ray defects and absent thumb, though the craniofacial findings are absent, and cardiac defects are present [87].
Condition | Face | Limb | Other | Genetics |
|---|---|---|---|---|
Frequencies of clinical characteristics are listed if available. GU: genitourinary; CNS: central nervous system. | ||||
Acrofacial dysostosis, Nager type | Micrognathia (93%) Ear malformation (79%) Downslanting palpebral fissures (63%) Orofacial cleft (40%) | Abnormal thumb (84%) Hypoplastic 5th finger (5%) Abnormal radius (67%) Abnormal ulna (13%) Clubfoot (16%) | Cardiac defect (14%) GU anomalies (8%) | SF3B4 |
Acrofacial dysostosis, Rodriguez type [10] | Micrognathia (100%) Ear malformation (90%) Hypertelorism (45%) Orofacial cleft (30%) | Abnormal thumb (100%) Hypoplastic 5th finger (35%) Absent radius (40%) Absent ulna (65%) Hypoplastic scapula (40%) Absent fibula (45%) Clubfoot (45%) Hypoplastic pelvic girdle (35%) | 11 ribs (60%) Cardiac defect (35%) GU anomalies (30%) CNS defect (30%) Lung hypoplasia (25%) | SF3B4 |
Acrofacial dysostosis, Guion-Almeida type [79] | Ear malformation (100%) Micrognathia (94%) Orofacial cleft (27%) | Thumb anomalies (19%) | Microcephaly (85%) Cardiac defect (32%) Esophageal atresia (50%) | EFTUD2 |
Acrofacial dysostosis, Miller type (Genee-Wiedemann) [80] | Orofacial cleft (77%) Micrognathia (75%) Eyelid anomalies (70%) Eyelid coloboma (20%) Slanting palpebral fissures (43%) External ear anomalies (64%) | Limb anomalies (100%) Absence/hypoplasia of 5th digit (89%) Hypoplasia of ulna/radius (52%) Foot anomalies (91%) | Vertebral anomalies (27%) Cardiac defect (16%) Genital anomalies (16%) | DHODH |
Roberts syndrome [81] | Hypertelorism (86%) Micrognathia (74%) Ear malformation (66%) Orofacial clefts (57%) Downslanting palpebral fissures (56%) | Phocomelia (100%) Upper and lower extremity (80%) Lower extremity (20%) | Growth retardation (100%) Microcephaly (95%) | ESCO2 (defective chromatid cohesion) |
Orofaciodigital syndrome, type II (Mohr syndrome) [82] | Orofacial cleft Lobulated tongue Ear malformation | Polydactyly in upper and lower extremities Bifid thumb/hallux | Cardiac defect CNS defect | NEK1? [83] |
Trisomy 18 [84] | Absent nasal bone (5%) Micrognathia (4%) Orofacial cleft (4%) | Limb defects (52%) Clenched hand (23%) Rocker-bottom feet (8%) Shortened or absent long bones (9%) | Cardiac defect (67%) CNS defect (67%) Omphalocele (21%) Growth restriction (30%) | Chromosome 18 |
Treacher-Collins [85] | Downslanting palpebral fissures (99%) Micrognathia (88%) Ear malformation (70%) Eyelid coloboma (63%) Orofacial clefts (21%) | Limb anomaly (1%) | Cardiac defect (11%) Microcephaly (3%) | TCOF1 POLR1C POLR1D |
Oculo-auriculo-vertebral spectrum (Goldenhar syndrome) [86] | Ear malformation (100%) including microtia (89%) and preauricular skin tag (44%) Eye anomalies (24%) including epibulbar dermoids (8%) Hemifacial microsomia including micrognathia (49%) Orofacial clefts (18%) | Unilateral radial defects (2%) | Vertebral anomalies (24%) Cardiac defect (28%) | Not known |
Holt–Oram syndrome [87] | None | Thumb anomalies (100%) with absent thumb (49%) Radial agenesis/hypoplasia (49%) Ulnar agenesis/hypoplasia (25%) | Cardiac defects (79%) | TBX5 |
Patients affected with Nager syndrome often have normal intelligence, though due to the otologic and mandibular abnormalities may have congenital conductive hearing loss, speech difficulties and upper airway obstruction [3]. Neonatal mortality in patients with Nager syndrome is 9-12% and often related to respiratory compromise (19,88). Therefore, prenatal diagnosis is crucial for patient counseling and to prepare a multidisciplinary team that can address airway obstruction at delivery. In infancy, patients may require tracheostomy for respiration and gastrostomy feeding until surgical correction of their mandibular abnormalities and orofacial clefts can be performed [66,88]. Approximately 80% of patients will exhibit conductive hearing loss, and will require hearing aids [19]. Limb anomalies can be addressed with surgical intervention or bracing. Patients often need physical therapy to address both speech difficulties as well as limb defects [66].
References
[1] Nager FR, de Reynier JP. Das Gehörorgan bei den angeborenen Kopfmissbildungen. Practica oto-rhino-laryngologica (Basel). 1948; 10 (suppl. 2): 1–128
[2] Benson CB, Pober BR, Hirsh MP, et al. Sonography of Nager acrofacial dysostosis syndrome in utero. J Ultrasound Med. 1988 Mar;7(3):163-7.
[3] Couyoumjian CA, Treadwell MC, Barr M. Prenatal sonographic diagnosis of Nager acrofacial dysostosis with unilateral upper limb involvement. Prenat Diagn. 2008 Oct;28(10):964-6.
[4] Petit F, Escande F, Jourdain AS, et al. Nager syndrome: confirmation of SF3B4 haploinsufficiency as the major cause. Clin Genet. 2014 Sep;86(3):246-51.
[5] Bernier FP, Caluseriu O, Ng S, et al. Haploinsufficiency of SF3B4, a component of the pre-mRNA spliceosomal complex, causes Nager syndrome. Am J Hum Genet. 2012 May 4;90(5):925-33.
[6] Czeschik JC, Voigt C, Alanay Y, et al. Clinical and mutation data in 12 patients with the clinical diagnosis of Nager syndrome. Hum Genet. 2013 Aug;132(8):885-98.
[7] Ulhaq ZS, Soraya GV, Istifiani LA, et al. SF3B4 Frameshift Variants Represented a More Severe Clinical Manifestation in Nager Syndrome. Cleft Palate Craniofac J. 2023 Aug;60(8):1041-1047.
[8] Rodríguez JI, Palacios J, Urioste M. New acrofacial dysostosis syndrome in 3 sibs. Am J Med Genet. 1990 Apr;35(4):484-9.
[9] McPherson E, Zaleski C, Ye Z, et al. Rodriguez syndrome with SF3B4 mutation: a severe form of Nager syndrome? Am J Med Genet A. 2014 Jul;164A(7):1841-5.
[10] Drivas TG, Taylor JA, Zackai EH. The final demise of Rodriguez lethal acrofacial dysostosis: A case report and review of the literature. Am J Med Genet A. 2019 Jun;179(6):1063-1068.
[11] Irving MD, Dimitrov BI, Wessels M, et al. Rodriguez acrofacial dysostosis is caused by apparently de novo heterozygous mutations in the SF3B4 gene. Am J Med Genet A. 2016 Dec;170(12):3133-3137.
[12] Norris RA, Scott KK, Moore CS, et al. Human PRRX1 and PRRX2 genes: cloning, expression, genomic localization, and exclusion as disease genes for Nager syndrome. Mamm Genome. 2000 Nov;11(11):1000-5.
[13] Opitz C, Stoll C, Ring P. Nager syndrome. Problems and possibilities of therapy. J Orofac Orthop. 2000;61(4):226-36.
[14] Denny AD, Talisman R, Hanson PR, et al. Mandibular distraction osteogenesis in very young patients to correct airway obstruction. Plast Reconstr Surg. 2001 Aug;108(2):302-11.
[15] Wang RY, Earl DL, Ruder RO, et al. Syndromic ear anomalies and renal ultrasounds. Pediatrics. 2001 Aug;108(2):E32.
[16] Meizner I, Mashiach R, Shalev J, et al. The 'tulip sign': a sonographic clue for in-utero diagnosis of severe hypospadias. Ultrasound Obstet Gynecol. 2002 Mar;19(3):250-3.
[17] Stelnicki EJ, Lin WY, Lee C, et al. Long-term outcome study of bilateral mandibular distraction: a comparison of Treacher Collins and Nager syndromes to other types of micrognathia. Plast Reconstr Surg. 2002 May;109(6):1819-25
[18] Margulis A, Patel PK, Daw JL, et al. Distraction osteogenesis of the mandible with an internal bioresorbable device. J Craniofac Surg. 2003 Sep;14(5):791-6.
[19] Herrmann BW, Karzon R, Molter DW. Otologic and audiologic features of Nager acrofacial dysostosis. Int J Pediatr Otorhinolaryngol. 2005 Aug;69(8):1053-9
[20] Heller JB, Gabbay JS, Kwan D, et al. Genioplasty distraction osteogenesis and hyoid advancement for correction of upper airway obstruction in patients with Treacher Collins and Nager syndromes. Plast Reconstr Surg. 2006 Jun;117(7):2389-98.
[21] Kleine-Hakala M, Hukki J, Hurmerinta K. Effect of mandibular distraction osteogenesis on developing molars. Orthod Craniofac Res. 2007 Nov;10(4):196-202.
[22] Spector JA, Warren SM, Singh SP, et al. Marriage of hard and soft tissues of the face revisited: when distraction meets microsurgery. Ann Plast Surg. 2007 Jul;59(1):1-5; discussion 5.
[23] Gürsoy S, Hukki J, Hurmerinta K. Five year follow-up of mandibular distraction osteogenesis on the dentofacial structures of syndromic children. Orthod Craniofac Res. 2008 Feb;11(1):57-64.
[24] Kaban LB, Seldin EB, Kikinis R, et al. Clinical application of curvilinear distraction osteogenesis for correction of mandibular deformities. J Oral Maxillofac Surg. 2009 May;67(5):996-1008.
[25] Schlieve T, Almusa M, Miloro M, et al. Temporomandibular joint replacement for ankylosis correction in Nager syndrome: case report and review of the literature. J Oral Maxillofac Surg. 2012 Mar;70(3):616-25.
[26] Arosarena OA, Hemme T. Management of soft palate agenesis in Nager syndrome with an elongated, superiorly based pharyngeal flap. Ear Nose Throat J. 2014 Oct-Nov;93(10-11):E1-5.
[27] Franchi G, Kadlub N, Diner PA, et al. Orbital soft tissue surgery for patients with Treacher-Collins or Nager syndrome. A new surgical approach with early correction of soft tissue: prospective study. Br J Oral Maxillofac Surg. 2015 May;53(5):421-5.
[28] Lean LL, King C. Use of the C-MAC adult D blade in paediatric patients with Nager syndrome. Anaesth Intensive Care. 2016 Sep;44(5):647-8.
[29] Sjogren PP, Gurgel RK, Park AH. Does canal wall down mastoidectomy benefit syndromic children with congenital aural stenosis? Int J Pediatr Otorhinolaryngol. 2016 Nov:90:200-203.
[30] Denu RA, Burkard ME. Synchronous Bilateral Breast Cancer in a Patient With Nager Syndrome. Clin Breast Cancer. 2017 Jun;17(3):e151-e153.
[31] Tay SY, Loh WS, Lim TC. A Case Report of Absent Epiglottis in Children With Nager Syndrome: Its Impact on Swallowing. Cleft Palate Craniofac J. 2017 Nov;54(6):754-757.
[32] Wu CC, Sakahara D, Imai K. Ankylosis of temporomandibular joints after mandibular distraction osteogenesis in patients with Nager syndrome: Report of two cases and literature review. J Plast Reconstr Aesthet Surg. 2017 Oct;70(10):1449-1456.
[33] Biskup NI, Pan BS, Elhadi-Babiker H, et al. Decannulation and Airway Outcomes With Maxillomandibular Distraction in Treacher Collins and Nager Syndrome. J Craniofac Surg. 2018 May;29(3):692-697.
[34] Simpson AM, Mehta ST, Siddiqi F, et al. Modified Lefort Distraction Osteogenesis for the Treatment of Nager Syndrome-Associated Midface Hypoplasia: Technique and Review. J Craniofac Surg. 2018 Sep;29(6):e621-e623.
[35] Karempelis P, Hagen M, Morrell N, et al. Associated syndromes in patients with Pierre Robin Sequence. Int J Pediatr Otorhinolaryngol. 2020 Apr;131:109842.
[36] Hodzic Z, Törnwall J, Leikola J, et al. Alloplastic Temporomandibular Joint Reconstruction in Congenital Craniofacial Deformities. J Craniofac Surg. 2021 Sep 1;32(6):e548-e551.
[37] Wenger TL, Wild KT, Zaniletti I, et al. Management and Outcomes of Neonates with Treacher Collins and Nager Syndromes. J Pediatr. 2025 Aug;283:114614.
[38] Bates AW, Hall CM, Morgan H, et al. Lethal acrofacial dysostosis, pre- and post-axial defects of the hands, and bilateral renal agenesis. Clin Dysmorphol. 2002 Jan;11(1):63-6.
[39] Dimitrov B, Balikova I, Jekova N, Vakrilova L, et al. Acrofacial dysostosis type Rodríguez. Am J Med Genet A. 2005 May 15;135(1):81-5.
[40] Miyawaki M, Higuchi R, Yoshikawa N. Rodriguez lethal acrofacial dysostosis syndrome with pulmonary hypoplasia. Pediatr Int. 2009 Aug;51(4):593-5.
[41] Ural UM, Ceylaner S. Rodriguez lethal acrofacial dysostosis syndrome with ambiguous genitalia. Taiwan J Obstet Gynecol. 2016 Aug;55(4):613-5.
[42] Wessels MW, Den Hollander NS, Cohen-Overbeek TE, et al. Prenatal diagnosis and confirmation of the acrofacial dysostosis syndrome type Rodriguez. Am J Med Genet. 2002 Nov 15;113(1):97-100.
[43] Sermer D, Quercia N, Chong K, et al. Acrofacial dysostosis syndrome type Rodriguez: prenatal diagnosis and autopsy findings. Am J Med Genet A. 2007 Dec 15;143A(24):3286-9.
[44] Gana S, Gentilin B, Bianchi V, et al. Prenatal phenotype of Nager syndrome and Rodriguez syndrome: variable expression of the same entity? Clin Dysmorphol. 2013 Oct;22(4):135-139.
[45] Marques F, Tenney J, Duran I, et al. Altered mRNA Splicing, Chondrocyte Gene Expression and Abnormal Skeletal Development due to SF3B4 Mutations in Rodriguez Acrofacial Dysostosis. PLoS Genet. 2016 Sep 13;12(9):e1006307.
[46] Kubota H, Noguchi Y, Urabe K, et al. Flexor digitorum longus accessorius in the club foot of an infant with Nager syndrome. Arch Orthop Trauma Surg. 2001;121(1-2):95-6.
[47] Groeper K, Johnson JO, Braddock SR, et al. Anaesthetic implications of Nager syndrome. Paediatr Anaesth. 2002 May;12(4):365-8.
[48] Scapoli L, Martinelli M, Pezzetti F, et al. Spontaneous expression of FRA3P in a patient with Nager syndrome. Am J Med Genet A. 2003 Apr 30;118A(3):293-5.
[49] Kavadia S, Kaklamanos EG, Antoniades K, et al. Nager syndrome (preaxial acrofacial dysostosis): a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004 Jun;97(6):732-8.
[50] Halonen K, Hukki J, Arte S, et al. Craniofacial structures and dental development in three patients with Nager syndrome. J Craniofac Surg. 2006 Nov;17(6):1180-7.
[51] Kahrom M, Abbaszadegan MR, Kahrom H, et al. Nager's acrofacial dysostosis with hypertrophic cardiomyopathy. Saudi Med J. 2006 Oct;27(10):1578-81.
[52] Ho AS, Aleshi P, Cohen SE, et al. Airway management in Nager Syndrome. Int J Pediatr Otorhinolaryngol. 2008 Dec;72(12):1885-8.
[53] Van Lierde KM, Luyten A, Mortier G, et al. Overall intelligibility, articulation, resonance, voice and language in a child with Nager syndrome. Int J Pediatr Otorhinolaryngol. 2011 Feb;75(2):270-6.
[54] Bernier FP, Caluseriu O, Ng S, et al. Haploinsufficiency of SF3B4, a component of the pre-mRNA spliceosomal complex, causes Nager syndrome. Am J Hum Genet. 2012 May 4;90(5):925-33.
[55] Davies GP, Johnson IJ. The first reported treatment of Nager syndrome associated hearing loss with bone-anchored hearing aids: case report. J Laryngol Otol. 2012 Jan;126(1):76-8.
[56] Lin JL. Nager syndrome: a case report. Pediatr Neonatol. 2012 Apr;53(2):147-50.
[57] Malik R, Goel S, Aggarwal S. Limbal dermoid in Nager acrofacial dysostosis: a rare case report. Indian J Ophthalmol. 2014 Mar;62(3):339-41.
[58] Pengelly RJ, Upstill-Goddard R, Arias L, et al. Resolving clinical diagnoses for syndromic cleft lip and/or palate phenotypes using whole-exome sequencing. Clin Genet. 2015 Nov;88(5):441-9.
[59] Rosa RF, Guimarães VB, Beltrão LA, et al. Nager syndrome and Pierre Robin sequence. Pediatr Int. 2015 Apr;57(2):e69-72.
[60] Chummun S, McLean NR, Anderson PJ, et al. The Craniofacial and Upper Limb Management of Nager Syndrome. J Craniofac Surg. 2016 Jun;27(4):932-7.
[61] Nair S, Agarwal P, Oak S, et al. Nager Syndrome with Eventration of Diaphragm: A Rare Presentation. Indian J Pediatr. 2016 Sep;83(9):1043-4.
[62] Cassina M, Cerqua C, Rossi S, et al. A synonymous splicing mutation in the SF3B4 gene segregates in a family with highly variable Nager syndrome. Eur J Hum Genet. 2017 Feb;25(3):371-375.
[63] Zhao J, Yang L. Broad-spectrum next-generation sequencing-based diagnosis of a case of Nager syndrome. J Clin Lab Anal. 2020 Sep;34(9):e23426.
[64] Cadieux-Dion M, Hughes S, Engleman K, et al. Nager syndrome in patient lacking acrofacial dysostosis: Expanding the phenotypic spectrum of SF3B4-related disease. Am J Med Genet A. 2021 May;185(5):1515-1518.
[65] Trindade PAK, Bueno PM, Scomparin L,et al. The role of double-step advancement genioplasty and bilateral coronoidectomy in Nager Syndrome: A case report. Spec Care Dentist. 2021 Jul;41(4):512-518.
[66] Marszałek-Kruk BA, Myśliwiec A, Lipowicz A, et al. Children with Rare Nager Syndrome-Literature Review, Clinical and Physiotherapeutic Management. Genes (Basel). 2023 Dec 24;15(1):29.
[67] Paladini D, Tartaglione A, Lamberti A, et l. Prenatal ultrasound diagnosis of Nager syndrome. Ultrasound Obstet Gynecol. 2003 Feb;21(2):195-7.
[68] Martinez R. “Nager syndrome.” TheFetus.net. https://thefetus.net/content/nager-syndrome-1, publish date 4/2004.
[69] Verrotti C, Benassi G, Piantelli G, et al. Acrofacial dysostosis syndromes: a relevant prenatal dilemma. A case report and brief literature review. J Matern Fetal Neonatal Med. 2007 Jun;20(6):487-90.
[70] Ansart-Franquet H, Houfflin-Debarge V, Ghoumid J, et al. Prenatal diagnosis of Nager syndrome in a monochorionic-diamniotic twin pregnancy. Prenat Diagn. 2009 Feb;29(2):187-9.
[71] Moreira Rios LT, Araujo Júnior E, Machado Nardozza LM, et al. Prenatal diagnosis of Nager syndrome in the third trimester of pregnancy and anatomopathological correlation. J Med Ultrason (2001). 2012 Oct;39(4):287-9.
[72] Castori M, Bottillo I, D'Angelantonio D, et al. A 22-Week-Old Fetus with Nager Syndrome and Congenital Diaphragmatic Hernia due to a Novel SF3B4 Mutation. Mol Syndromol. 2014 Aug;5(5):241-4.
[73] Lund IC, Vestergaard EM, Christensen R, et al. Prenatal diagnosis of Nager syndrome in a 12-week-old fetus with a whole gene deletion of SF3B4 by chromosomal microarray. Eur J Med Genet. 2016 Jan;59(1):48-51.
[74] Hayata K, Masuyama H, Eto E, et al. A Case of Nager Syndrome Diagnosed Before Birth. Acta Med Okayama. 2019 Jun;73(3):273-277.
[75] Drozniewska M, Kilby MD, Vogt J, et al. Second-trimester prenatal diagnosis of Nager syndrome with a deletion including SF3B4 detected by chromosomal microarray. Clin Case Rep. 2020 Feb 6;8(3):508-511.
[76] Drendel HM, Wilson C, Sagaribay P, et al. Prenatal diagnosis of Acrofacial Dysostosis type 1 (Nager syndrome) by chromosomal microarray at the exon level. Cancer Genetics. 2021 Mar;252–253:S5-S5. Supplement 1.
[77] Veduta A, Duta S, Ciobanu AM, et al. Fetal Skeletal Dysplasias that Involve the Face: Binder Syndrome and Nager Syndrome. Maedica (Bucur). 2021 Mar;16(1):140-144.
[78] Waggoner DJ, Ciske DJ, Dowton SB, et al. Deletion of 1q in a patient with acrofacial dysostosis. Am J Med Genet. 1999 Feb 12;82(4):301-4.
[79] Lehalle D, Gordon CT, Oufadem M, et al. Delineation of EFTUD2 haploinsufficiency-related phenotypes through a series of 36 patients. Hum Mutat. 2014 Apr;35(4):478-85.
[80] van Roey VL, Ombashi S, Kaymaz I, al. Unveiling the Phenotypic Spectrum of Miller Syndrome: A Systematic Review. J Craniofac Surg. 2025 May 19;36(8):e1243-e1247.
[81] Vega H, Trainer AH, Gordillo M, et al. Phenotypic variability in 49 cases of ESCO2 mutations, including novel missense and codon deletion in the acetyltransferase domain, correlates with ESCO2 expression and establishes the clinical criteria for Roberts syndrome. J Med Genet. 2010 Jan;47(1):30-7.
[82] Balci S, Guler G, Kale G, et al. Mohr Syndrome in Two Sisters: Prenatal Diagnosis in a 22-week-old Fetus with Post-mortem Findings in Both. Prenat Diagn. 1999 Sep;19(9):827-31.
[83] Monroe GR, Kappen IF, Stokman MF, et al. Compound heterozygous NEK1 variants in two siblings with oral-facial-digital syndrome type II (Mohr syndrome). Eur J Hum Genet. 2016 Dec;24(12):1752-1760
[84] Becker DA, Tang Y, Jacobs AP, et al. Sensitivity of prenatal ultrasound for detection of trisomy 18. J Matern Fetal Neonatal Med. 2019 Nov;32(22):3716-3722
[85] Vincent M, Geneviève D, Ostertag A, et al. Treacher Collins syndrome: a clinical and molecular study based on a large series of patients. Genet Med. 2016 Jan;18(1):49-56.
[86] Barisic I, Odak L, Loane M, et al. Prevalence, prenatal diagnosis and clinical features of oculo-auriculo-vertebral spectrum: a registry-based study in Europe. Eur J Hum Genet. 2014 Aug;22(8):1026-33.
[87] Barisic I, Boban L, Greenlees R, et al. Holt Oram syndrome: a registry-based study in Europe. Orphanet J Rare Dis. 2014 Oct 25:9:156.
[88] Danziger I, Brodsky L, Perry R, et al. Nager's acrofacial dysostosis. Case report and review of the literature. Int J Pediatr Otorhinolaryngol. 1990 Dec;20(3):225-40.
Discussion Board
Winners

Javier Cortejoso Spain Physician

Padman KG United Kingdom Sonographer

Iuliia Iudina Russian Federation Physician

belen garrido Spain Physician

Andrii Averianov Ukraine Physician

Alexandr Krasnov Ukraine Physician
Carlos Orellana Venezuela Physician

Mayank Chowdhury India Physician

Vladimir Lemaire United States Physician

Boujemaa Oueslati Tunisia Physician

Tatiana Koipish Belarus Physician

carlos lopez Venezuela Physician

Peter conner Sweden Physician
Marianovella Narcisi Italy Physician

Amparo Gimeno Spain Physician

Elena Andreeva Russian Federation Physician
Muradiye YILDIRIM Turkey Physician

ALBANA CEREKJA Italy Physician

Eti Zetounie Israel Sonographer

Murat Cagan Turkey Physician

Sonio Sonio France AI

ANA PAULA PASSOS Brazil Physician

Ionut Valcea Romania Physician
Đặng Mai Quỳnh Viet Nam Physician

Hien Nguyen Van Viet Nam Physician
TEJAS TAMHANE India Physician
Zuzana Briešková Slovakia Physician

Anette Beverdam Netherlands Sonographer

Annette Reuss Germany Physician

Vu The Anh Viet Nam Physician

shay kevorkian Israel Physician

Ismail Guzelmansur Turkey Physician

zozo sichala Zambia radiology technologist
philip pattyn Belgium Physician

Denys Saitarly Israel Physician

Le Tien Dung Viet Nam Physician

Tetiana Ishchenko Ukraine Physician

Le Duc Viet Nam Physician

Philippe Viossat Antarctica Consultant

Hana Habanova Slovakia Physician
Petra Tallova Slovakia Physician

ASHLEA HARDIN United States Sonographer

Dubyanskaya Yuliya Russian Federation Physician
Dang Thinh Nguyen Viet Nam Physician
Maria Bulanova Russian Federation Physician

Anne Janke Germany Physician

Mert Eyupoglu Turkey Physician

MEHMET AYGÜN Turkey Physician

Gulten Rafibeyli Azerbaijan Physician
Truong Tran Duc Viet Nam Physician

Hilal gülsüm Turan Özsoy Turkey Physician

Ayten Sadigova Azerbaijan Physician

Gulsum Mammadova Azerbaijan Physician
Aynur Garibova Azerbaijan Physician

Ulviyya Jafarova Azerbaijan Physician