Case of the Week #615
(1) Astha Hospital, Deesa, Gujarat, India; (2) Hospital Recoletas Campo Grande, Valladolid, Spain
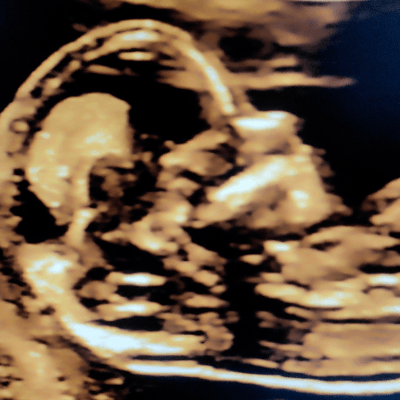
22-year-old primigravida presented for a routine scan at 20 weeks of gestation. Our images demonstrated the following findings:


View the Answer Hide the Answer
Answer
We present a case of megalourethra. The patient refused further investigations and opted for termination of pregnancy at a remote center.
Our prenatal images demonstrated a dilated penile urethra with keyhole type of urinary bladder and bilateral renal pelvis dilatation.

Discussion
Congenital urogenital anomalies are among the most common organ system abnormalities in the neonate. Its combined global incidence is 8.4 per 1000 live births [1]. Urethral anomalies may vary from an insignificant incidental finding to severe obstructive variants incompatible with life. The more severe types that lead to upper tract dilatation or renal cystic dysplasia are most easily diagnosed prenatally [2].
Congenital megalourethra is a urogenital anomaly characterized by a cystic dilatation and elongation of the penile urethra resulting from the absence and hypoplasia of penile erectile tissues [3]. The term was originally described by Nesbitt who reported a rare congenital malformation in a one-month-old child characterized by diffuse dilation of the penile urethra without distal obstruction [4]. During development, canalization and connection between the penile and glandular segments of the urethra is completed at 18 weeks of gestation. Stephens and Fortune [5] postulated that in patients with megalourethra, canalization of the urethra of the penile glans is delayed, causing a temporary obstruction that leads to compression and arrested development of the corpus spongiosum, and at times also the corpora cavernosa. The erectile tissues differentiate from the mesodermal columns during the seventh week of development, thus other theories propose a very early failure of mesenchymal development as cause of megalourethra [6,7].
Dorairajan described two forms of congenital megalourethra based on the shape and extent of associated defects of erectile tissue: scaphoid megalourethra and fusiform megalourethra [8]. In the scaphoid type, which is the most common and least severe form, only the corpus spongiosum is absent. In the fusiform variety, the corpora cavernosa and the corpus spongiosum are absent, thus the urethra is only covered by skin and subcutaneous tissue. The severity of the urethral anomaly is related to the duration of obstruction during embryogenesis, with fusiform megalourethra suffering a more delayed canalization. In fact, both variants represent points along a continuum, and there may be patients with intermediate phenotypes [7,9]. Dorairajan’s classification describes morphology and does not stratify patients based on prognosis. Ansalem et al [10] propose a new classification that categorizes patients on whether the hypoplasia/absence of the corpora is the result of an initial absence of corporal tissue (primary type) or secondary to an obstruction (secondary or obstructive type). The primary type is associated with normal amniotic fluid volume and better outcomes, although can become obstructive if blocked by debris and result in oligohydramnios later in gestation.
Prenatal sonographic diagnosis of megalourethra has been reported on several occasions, usually as isolated cases. The first prenatal case was reported in 1989 by Benacerraf et al [11], and the earliest diagnosis was made at 13 weeks of gestation [12,13]. The prenatal diagnosis is established by the presence of a blind cystic structure between the fetal legs or arising from the perineum, which corresponds to the elongated and/or distended phallic structure. It is advisable to use color Doppler sonography to rule out the presence of a loop or cyst of the umbilical cord, which can be mistakenly considered as a dilated urethra [10, 14]. During fetal life, megalourethra can lead to lower urinary tract obstruction (LUTO) which manifests with megacystis, bladder wall hypertrophy, hydroureters, and hydronephrosis. Additionally, there can be bilateral renal dysplasia, oligo- or anhydramnios, and Potter syndrome-like manifestations in the newborn [10]. Fusiform megalourethra, which invariably is associated with signs of urinary tract dilatation, is more likely detected prenatally than scaphoid megalourethra, which tends to be less severe [14]. Megalourethra and prune belly syndrome may be on a spectrum of defective mesenchymal development, with megalourethra on the less severe side and prune belly syndrome on the more severe side with findings of deficient abdominal wall musculature, cryptorchidism, and urinary obstruction [6,15]. The mesenchymal defect could explain other severe mesenchymal anomalies associated with congenital megalourethra such as cloacal anomalies, imperforate anus, refluxing megaureters, and the VACTERL (Vertebral anomalies, Anal atresia, Cardiac anomalies, TracheoEsophageal fistula, Renal anomalies, and Limb anomalies) association [13,16,17].
The worst prognosis occur in cases with severe oligohydramnios/anhydramnios, earlier gestational age at decreased amniotic fluid volume, bilateral hydroureters and hydronephrosis and presence of renal cysts [18]. Prenatal spontaneous resolution of the megalourethra can occur in up to 10% of cases, resulting in births of healthy children [19-21]. Several authors have performed fetal bladder drainages assessing urinary chemical analysis to determine renal function, assess prognosis and counsel patients [19,22,23], but the role of this invasive procedure remains questionable since the prognosis based on amniotic fluid volume is reliable [21]. Different therapeutic options have been considered, such as vesicocentesis [19, 23, 24], ultrasound guided puncture or aspiration of the dilated urethra [13, 25], vesicoamniotic shunting [10, 18], and fetoscopic urethral meatotomy [26, 27].
All live-born infants with this malformation manifest voiding dysfunction and sexual disorders, in addition to complications of lower urinary tract obstruction which may include oligohydramnios, pulmonary hypoplasia and chronic progressive renal failure [10]. The mortality rate associated with megalourethra has been reported to be as high as 66% for fusiform and 13% for scaphoid types [7], partly due to a high rate of extraurinary malformations.
When congenital megalourethra is suspected on ultrasound, the differential diagnosis may include posterior urethral valve, urethral atresia, web, or diverticulum, prune belly syndrome, megacystis-microcolon-intestinal-hypoperistalsis syndrome, and cloacal anomalies [28].
References:
[1] Huang X, Tang J, Chen M, et al. Sex difference and risk factors in burden of urogenital congenital anomalies from 1990 to 2019. Sci Rep. 2023 Aug 22;13(1):13656.
[2] Rauch MK, Gosalbez R, and Zaleski CG. Congenital Anomalies of the Urethra. In: Jafri SZH, Diokno AC, and Amendola MA, ed. Lower Genitourinary Radiology. Imaging and Intervention. Springer-Verlag New York, Inc. 1998; pages 333-351.
[3] Hakimi T. Congenital megalourethra. Ann Med Surg (Lond). 2022 Nov 17;84:104926
[4] Nesbitt TE. Congenital megalourethra. J Urol. 1955 May;73(5):839-42.
[5] Stephens FD, Fortune DW. Pathogenesis of megalourethra. J Urol. 1993 Jun;149(6):1512-6.
[6] Lockhart JL, Reeve HR, Krueger RP, et al. Megalourethra. Urology. 1978 Jul;12(1):51-4.
[7] Jones EA, Freedman AL, Ehrlich RM. Megalourethra and urethral diverticula. Urol Clin North Am. 2002 May;29(2):341-8, vi.
[8] Dorairajan T. Defects of spongy tissue and congenital diverticula of the penileurethra, ANZ J. Surg. 32 (1963) 209–214.
[9] Appel RA, Kaplan GW, Brock WA, et al. Megalourethra. J Urol. 1986 Apr;135(4):747-51.
[10] Amsalem H, Fitzgerald B, Keating S, et al. Congenital megalourethra: prenatal diagnosis and postnatal/autopsy findings in 10 cases. Ultrasound Obstet Gynecol. 2011 Jun;37(6):678-83.
[11] Benacerraf BR, Saltzman DH, Mandell J. Sonographic diagnosis of abnormal fetal genitalia. J Ultrasound Med. 1989 Nov;8(11):613-7.
[12] Lam YH, Tang MH. Sonographic diagnosis of congenital megalourethra at 13 weeks of gestation. Ultrasound Obstet Gynecol. 2000 Nov;16(6):585-6.
[13] Krapp M, Geipel A, Germer U, et al. First-trimester sonographic diagnosis of distal urethral atresia with megalourethra in VACTERL association. Prenat Diagn. 2002 May;22(5):422-4.
[14] Sepulveda W, Elorza C, Gutierrez J, et al. Congenital megalourethra: outcome after prenatal diagnosis in a series of 4 cases. J Ultrasound Med. 2005 Sep;24(9):1303-8.
[15] Fisk NM, Dhillon HK, Ellis CE, et al. Antenatal diagnosis of megalourethra in a fetus with the prune belly syndrome. J Clin Ultrasound. 1990 Feb;18(2):124-8.
[16] Chao AS, Chang YL, Hsieh PC. Prenatal diagnosis of congenital megalourethra with imperforate anus. BMC Pediatr. 2019 Apr 23;19(1):123.
[17] Ardiet E, Houfflin-Debarge V, Besson R, et al. Prenatal diagnosis of congenital megalourethra associated with VACTERL sequence in twin pregnancy: favorable postnatal outcome. Ultrasound Obstet Gynecol. 2003 Jun;21(6):619-20.
[18] Moaddab A, Sananes N, Hernandez-Ruano S, et al. Prenatal Diagnosis and Perinatal Outcomes of Congenital Megalourethra: A Multicenter Cohort Study and Systematic Review of the Literature. J Ultrasound Med. 2015 Nov;34(11):2057-64
[19] Nijagal A, Sydorak RM, Feldstein VA, et al. Spontaneous resolution of prenatal megalourethra. J Pediatr Surg. 2004 Sep;39(9):1421-3.
[20] Torcia F, Salvatori MM, Valente A, et al. Spontaneous in utero second-trimester resolution of isolated mild fetal megalourethra. J Ultrasound Med. 2007 Nov;26(11):1625-8.
[21] Wax JR, Pinette MG, Landes A, et al. Prenatal sonographic diagnosis of congenital megalourethra with in utero spontaneous resolution. J Ultrasound Med. 2009 Oct;28(10):1385-8.
[22] Sepulveda W, Berry SM, Romero R, et al. Prenatal diagnosis of congenital megalourethra. J Ultrasound Med. 1993 Dec;12(12):761-6
[23] Perrotin F, Ayeva-Derman M, Lardy H, et al. Prenatal diagnosis and postnatal outcome of congenital megalourethra. Report of two cases. Fetal Diagn Ther. 2001 Mar-Apr;16(2):123-8.
[24] Asma B, Jumana B. Megalourethra: a case report managed with a single intrauterine bladder aspiration. Australas J Ultrasound Med. 2012 Feb;15(1):18-23.
[25] Simma B, GaBner I, Brezinka C, et al. Complete prenatal urinary tract obstruction caused by congenital megalourethra. J Clin Ultrasound. 1992 Mar-Apr;20(3):197-9.
[26] Migliorelli F, Martínez JM, Gómez O, et al. Successful Fetoscopic Surgery to Release a Complete Obstruction of the Urethral Meatus in a Case of Congenital Megalourethra. Fetal Diagn Ther. 2015;38(1):77-80.
[27] Cruz-Martínez R, Martínez-Rodríguez M, Gámez-Varela A, et al. Fetoscopic urethral meatotomy in fetuses with lower urinary tract obstruction by congenital megalourethra. Prenat Diagn. 2021 May;41(6):772-777.
[28] Anh DD, Nguyen HT, Meagher S, et al. Prenatal diagnosis of congenital megalourethra in the second trimester of pregnancy. J Ultrason. 2019 Dec;19(79):302-304.
Discussion Board
Winners

Dianna Heidinger United States Sonographer

belen garrido Spain Physician

Andrii Averianov Ukraine Physician

Ana Ferrero Spain Physician

Mayank Chowdhury India Physician

Aysegul Ozel Turkey Physician
Marianovella Narcisi Italy Physician

Elena Andreeva Russian Federation Physician

Eti Zetounie Israel Sonographer

Deval Shah India Physician

gholamreza azizi Iran, Islamic Republic of Physician

abdullah sarıyıldırım Turkey Physician

mohamed ateya Egypt Physician

Dr.Umashankar Kawlas India Physician

Rupal Sasani India Physician

Tetiana Ishchenko Ukraine Physician

Costin Radu Lucian Romania Physician
Gaurav Sharma India Physician

Hetal Patel India Physician

Dr Dhara patel India Physician

Dr Mayur C Trivedi India Physician
Jagdish Suthar India Physician

DR RAJESH KAMBLE India Physician

Svetlana Bakhtiyarov United States Sonographer

Hân Đỗ Viet Nam Physician

Akhani Madhvi India Physician

Valerie Finkelstein United States Sonographer

Amrita Thakkar India Physician

Dhruvkumar Lakhani India Physician

Dr Ashish Gosai India Physician

Robert Brawura-Biskupski-Samaha Poland Physician

Dhruv Patel India Physician