Case of the Week #596
(1) Vinmec Times City Hospital, Hanoi, Vietnam; (2) Kasr Al Ainy teaching hospital, Egypt; (3) St Mary's Medical Center, San Francisco, USA
This was the second pregnancy of a 27-year-old woman with a healthy previous child, who was referred to our center at 23 weeks of gestation. First trimester screening showed a nuchal translucency of 1.6mm and non-invasive prenatal testing was low risk for trisomy 13, 18, 21.

View the Answer Hide the Answer
Answer
We present a case of Pallister–Killian syndrome with subtle signs detected in late second trimester ultrasound.
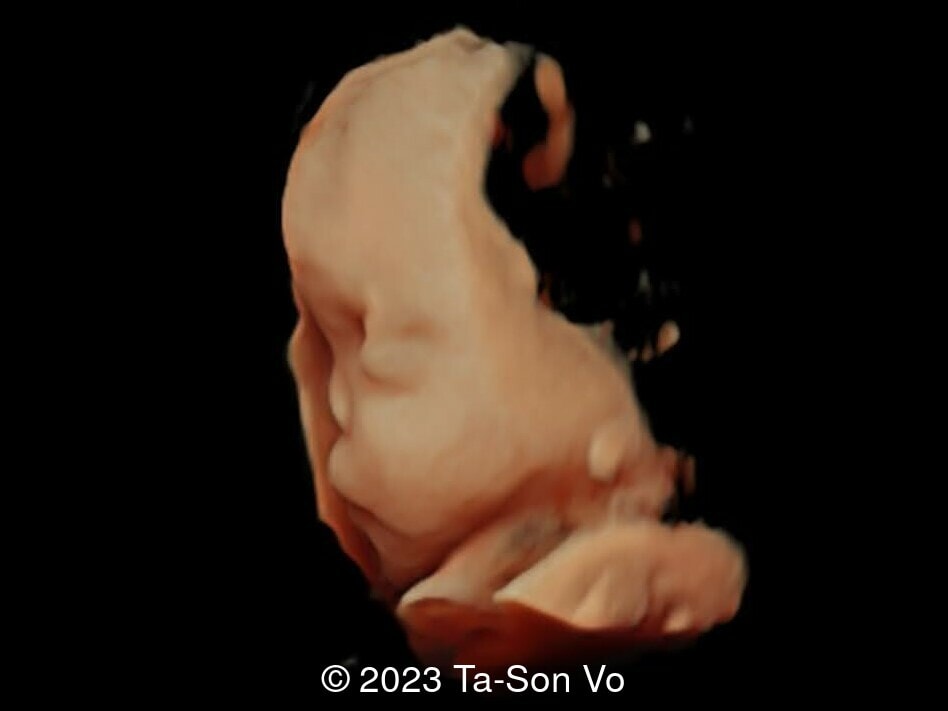
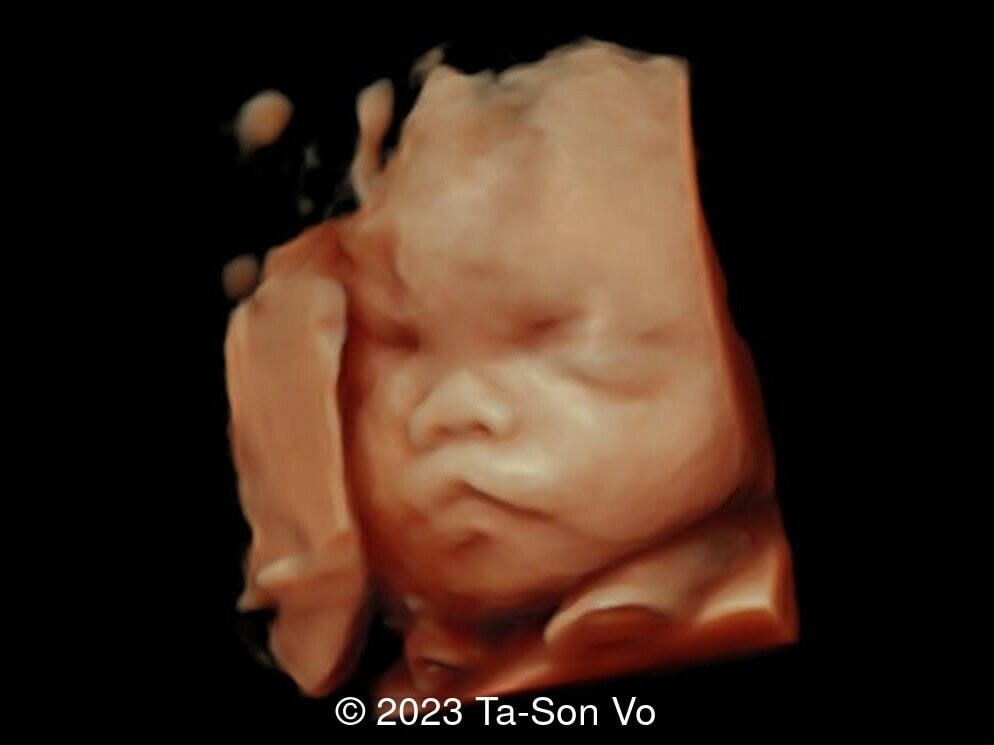
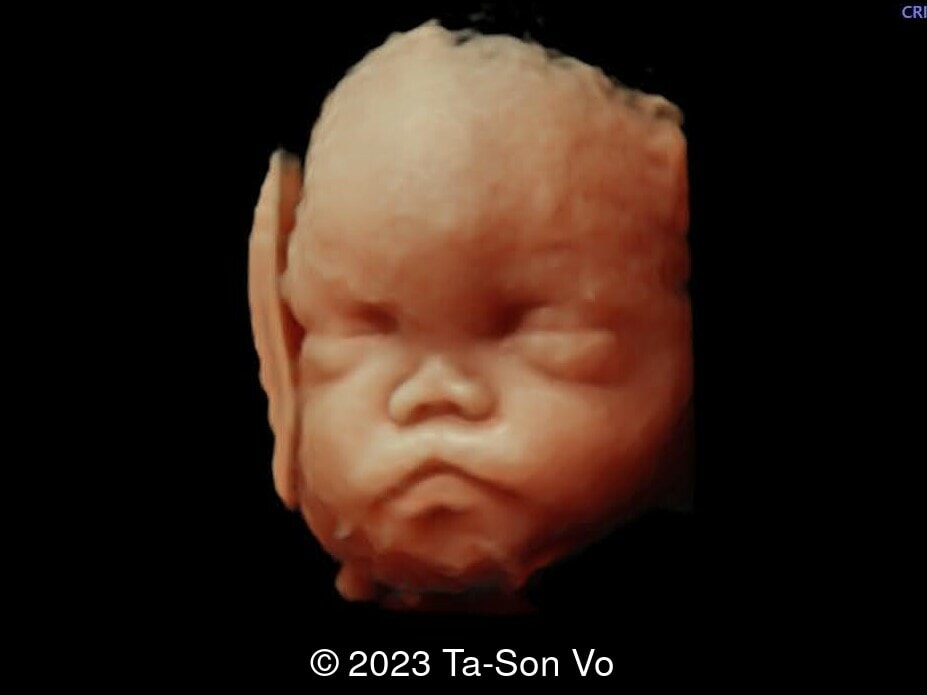
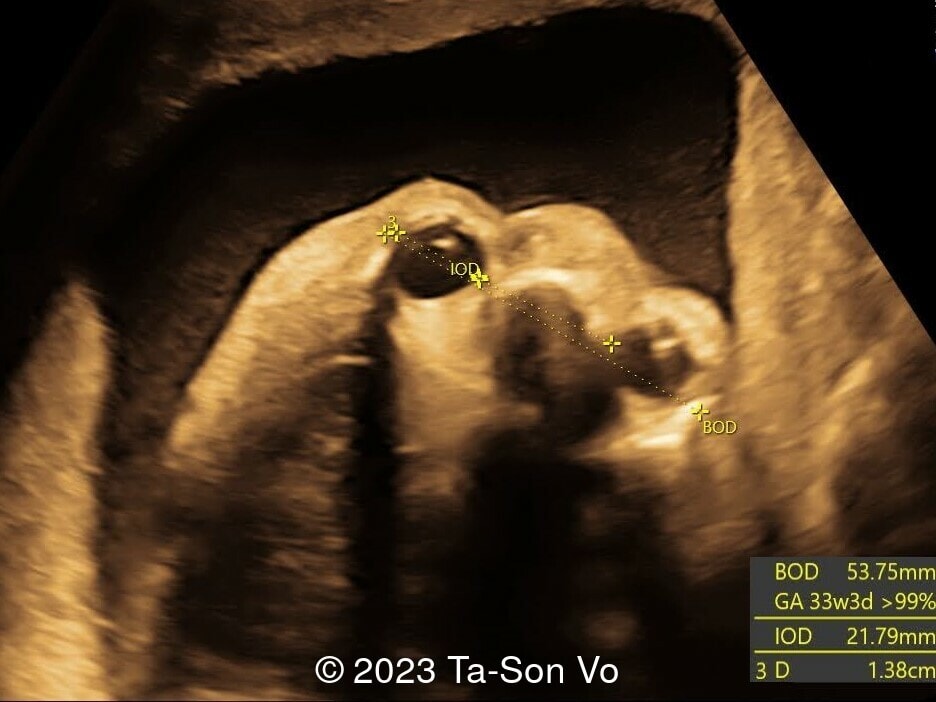
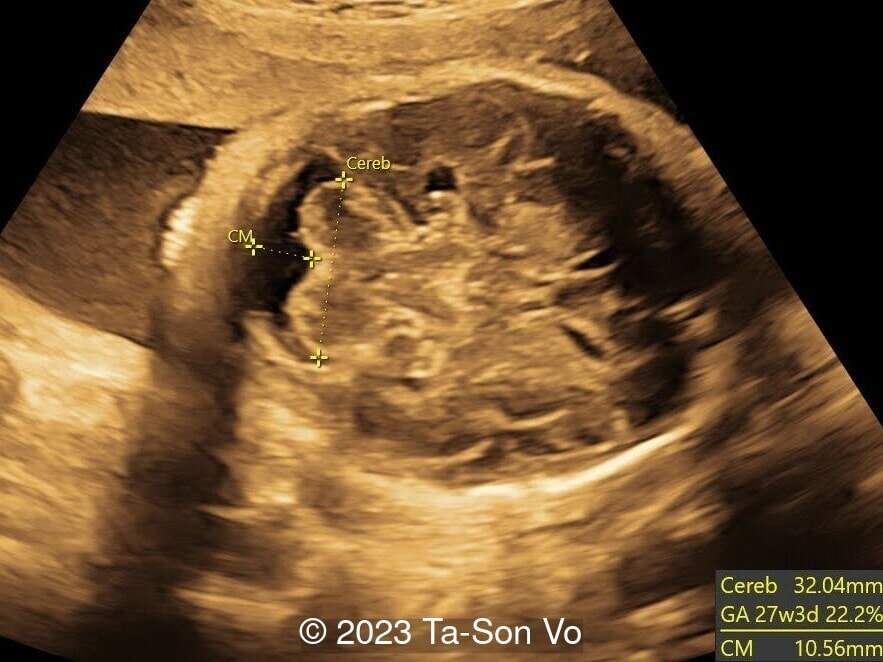
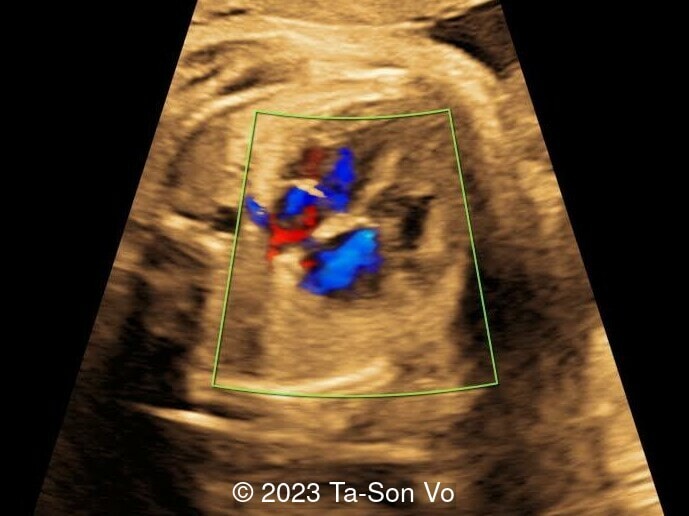
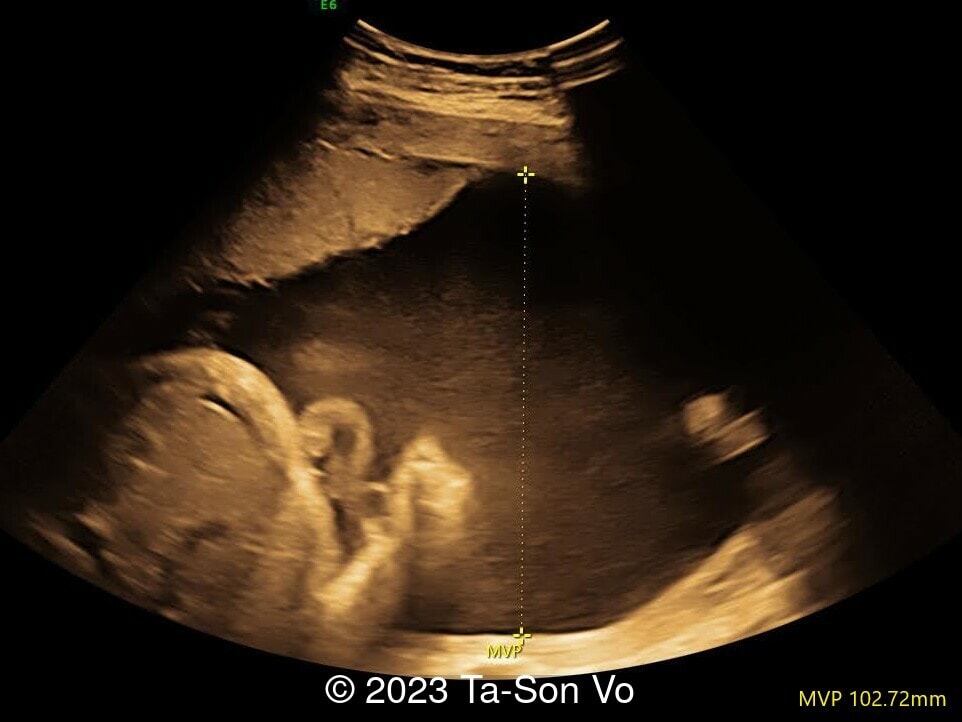
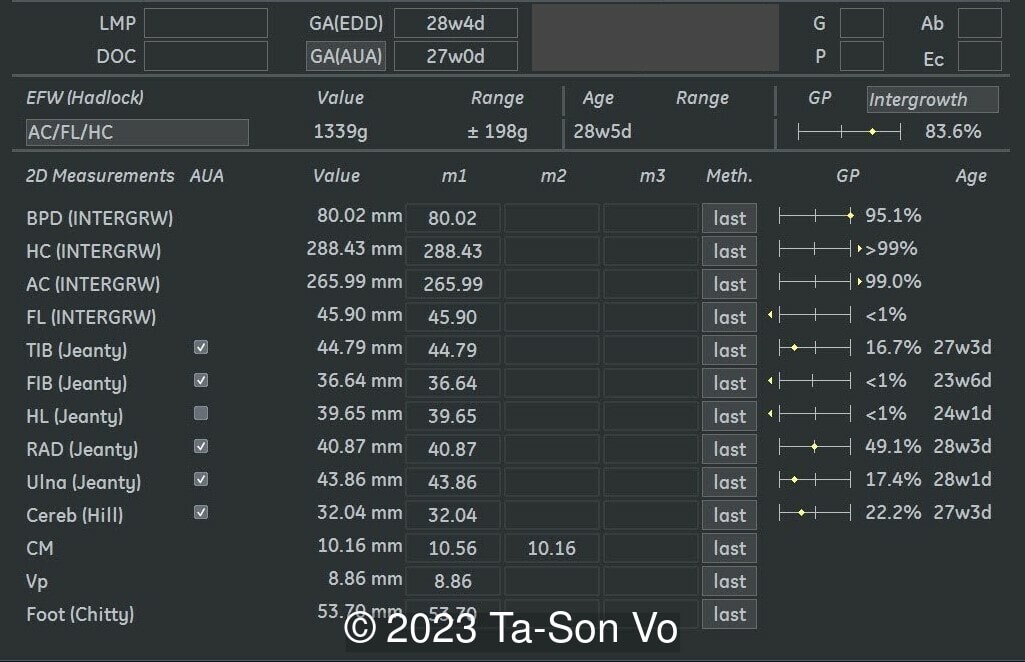
Level 2 ultrasound at 23 weeks and 28 weeks of gestation revealed thick nuchal and prenasal skin (Image 1), frontal bossing (Image 1,2), facial dysmorphism with a short nose, anteverted nostrils, flat nasal bridge, and long philtrum (Image 3,4), hypertelorism (Image 5), mega cisterna magna (Image 6) and polyhydramnios (Image 7). There was neither congenital diaphragmatic hernia nor cardiac defects with only mild tricuspid regurgitation (Image 8). Fetal biometry demonstrates a large head with rhizomelic limb shortening (Image 9).
Amniocentesis with karyotyping and copy number variation (CNV) sequencing was performed and showed a 33.9Mb duplication at chromosome 12p13.33p11.1 (arr[GRCH38] 12p13.33p11.1 (90834_33947065) x 4), which is classified as a pathogenic variant. The final report of karyotype Giemsa banding was mos 47,XX,i(12)(p10)[16]/46,XX[14]. These findings were consistent with clinical diagnosis of Pallister–Killian syndrome. After genetic counselling, the parents opted for termination of pregnancy.
Discussion
Pallister-Killian syndrome (PKS) is a rare genetic disorder usually caused by a tetrasomy of chromosome p12. The presence of two extra copies of the short arm of chromosome 12 occurs through a supernumerary isochromosome 12p and is often present in a tissue-limited mosaic state. Rarely, the Pallister-Killian phenotype can be present due to 12p duplication caused by an unbalanced translocation and resulting in trisomy 12p [1]. Reported cases of Pallister-Killian syndrome have been sporadic [2]. Most people with this disorder have severe to profound intellectual disability, seizures, hearing loss, dysmorphic facial features, pigmentary skin anomalies, as well as major organ malformations such as congenital diaphragmatic hernia and congenital heart disease [3]. Other cases are mild [4]. The incidence is approximately 5.1 per 1,000,000 live births [5].
Pallister-Killian syndrome is often incidentally detected based on nonspecific prenatal ultrasound abnormalities or advanced maternal age [2]. It was first diagnosed prenatally in 1987 via amniocytes as a tetrasomy of 12p [7]. Ultrasound findings can include facial dysmorphism characterized by frontal bossing, hypertelorism, long philtrum, increased prenasal thickness, flattened facial profile, and broad nasal bridge. Additionally, there can be increased nuchal translucency, thickened nuchal fold, rhizomelic shortening of the limbs, polyhydramnios, congenital diaphragmatic hernia, cerebral ventriculomegaly and cardiac anomalies [2,6,8-9].
In a study presenting the prenatal ultrasounds in four fetuses affected with Pallister-Killian syndrome and reviewing the literature, polyhydramnios was the most frequent anomaly accounting for 48% (94/194). Rhizomelic limb shortening occurred in 26% (51/194), and fetal macrosomia was present in 15% (29/194) [8]. While congenital diaphragmatic hernia is commonly described in Pallister-Killian syndrome, several case series reviewing prenatal findings do not identify this defect [6,8]. In a large case series reviewing 114 cases of Pallister-Killian syndrome, only 5% had congenital diaphragmatic hernia, though these cases were infants that survived the perinatal period [2]. In a literature review, the rate of congenital diaphragmatic hernia was 27%, though the true number is probably somewhere in between [2]. This reflects the diversity of clinical expression of Pallister-Killian syndrome.
Diagnosis is frequently missed due to the tissue-limited mosaicism, which refers to the differences in chromosome between different tissues in the same individual. The mosaicism rates of skin fibroblasts, amniotic cells or chorionic villus cells are higher than that of rapidly dividing lymphocytes [10], however the supernumerary marker chromosomes decrease in amniocytes during culturing [11]. As a result, several methods are used for prenatal diagnosis including single nucleotide polymorphism array (SNP array), copy number variants sequencing (CNV-seq), and fluorescence in situ hybridization (FISH) [6,8].
At birth, infants with Pallister-Killian syndrome show dysmorphic facial features, coarse facies, depressed nasal bridge, hearing loss, pigmented abnormalities of the skin, seizures, joint contractures, and hypotonia. Postnatally, brain MRI may show polymicrogyria (with cases reported to have unilateral polymicrogyria associated with calcifications), enlarged cisterna magna, enlarged lateral ventricles, white matter abnormalities, and thin corpus callosum [12].
In conclusion, increasing awareness and knowledge of the subtle prenatal ultrasound findings of Pallister-Killian syndrome is helpful for the early detection and counselling of patients. When femur shortening, polyhydramnios and macrosomia are observed together, they are highly indicative of Pallister-Killian syndrome [2]. As there is no correlation between the proportion of abnormal cells and prenatal ultrasound findings or outcomes [6], mosaism cannot be used to predict severity. Pallister-Killian syndrome infants with major structural anomalies such as congenital diaphragmatic hernia and congenital heart disease often do not survive, which can be helpful in counselling patients [2].
References
[1] Izumi K, Conlin LK, Berrodin D, et al. Duplication 12p and Pallister-Killian syndrome: a case report and review of the literature toward defining a Pallister-Killian syndrome minimal critical region. Am J Med Genet A. 2012 Dec;158A(12):3033-45.
[2] Salzano E, Raible SE, Kaur M, et al. Prenatal profile of Pallister-Killian syndrome: Retrospective analysis of 114 pregnancies, literature review and approach to prenatal diagnosis. Am J Med Genet A. 2018 Dec;176(12):2575-2586.
[3] Jamuar S, Lai A, Unger SH and Nishimura C. Clinical and radiological findings in Pallister–Killian syndrome. Eur J Med Genet. 2012 Mar;55(3):167-72.
[4] Bielanska MM, Khalifa MM, Duncan AM. Pallister-Killian syndrome: a mild case diagnosed by fluorescence in situ hybridization. Review of the literature and expansion of the phenotype. Am J Med Genet. 1996 Oct 16;65(2):104-8.
[5] Blyth M, Maloney V, Beal S, et al. Pallister-Killian syndrome: a study of 22 British patients. J Med Genet. 2015 Jul;52(7):454-64.
[6] Wu X, Xie X, Su L, et al. Prenatal diagnosis of Pallister-Killian syndrome and literature review. J Cell Mol Med. 2021 Sep;25(18):8929-8935.
[7] Steinbach P, Rehder H. Tetrasomy for the short arm of chromosome 12 with accessory isochromosome (+i(12p)) and a marked LDH-B gene dosage effect. Clin Genet. 1987 Jul;32(1):1-4.
[8] Wang T, Ren C, Dan Chen, et al. Prenatal diagnosis of Pallister-Killian syndrome using cord blood samples. Mol Cytogenet. 2019 Aug 30:12:39
[9] Abad DA, Gabarre JA, Izquierdo AM, Lopez-Sanchez C, Garcia-Martinez V. Pallister-Killian Syndrome Presenting With a Complex Congenital Heart Defect and Increased Nuchal Translucency. American institute of Ultrasound in Medicine 2006; 25: 1475-1480.
[10] Takakuwa K, Hataya I, Arakawa M, et al. A case of mosaic tetrasomy 12p (Pallister-Killian Syndrome) diagnosed prenatally: comparison of chromosome analyses of various cells obtained from the patient. Am J Perinatol. 1997 Nov;14(10):641-3.
[11] Polityko AD, Goncharova E, Shamgina L, et al. Pallister-Killian syndrome: rapid decrease of isochromosome 12p frequency during amniocyte subculturing. Conclusion for strategy of prenatal cytogenetic diagnostics. J Histochem Cytochem. 2005 Mar;53(3):361-4.
[12] Hiraiwa A, Matsui K, Nakayama Y, Komatsubara T, Magara SH, Kobayashi Y et al. Polymicrogyria with calcification in Pallister-Killian syndrome detected by microarray analysis. Brain and development March 2021; 43 (3): 448-453.
Discussion Board
Winners

Javier Cortejoso Spain Physician

Pawel Swietlicki Poland Physician

Andrii Averianov Ukraine Physician

Ana Ferrero Spain Physician

Alexandr Krasnov Ukraine Physician

Nutan Thakur India Physician

Nguyen Luân Viet Nam Physician

Ionut Valcea Romania Physician
Martina Vagaská Slovakia Physician

Megha V Oman Physician

Dr Ricecake Viet Nam Physician

Nguyễn Lê Hoàng Viet Nam Physician

Tetiana Ishchenko Ukraine Physician