Case of the Week #569
Associate professor OB/GYN Zagazig university, Egypt
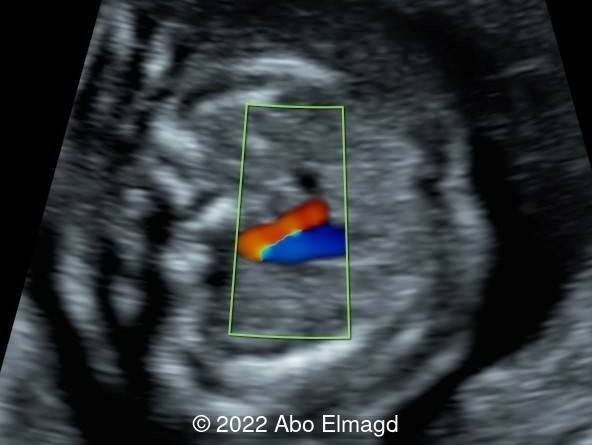
View the Answer Hide the Answer
Answer
Answer
Our ultrasound scan revealed critical aortic stenosis. The fetus was delivered at 36 weeks gestation and died at 24 hours of age.
-
Video 1: Abnormal four-chamber view. The left ventricle is dilated and globular with reduced contractility.
-
Video 2: The left ventricular wall was thickened and echogenic indicating endocardial fibroelastosis.
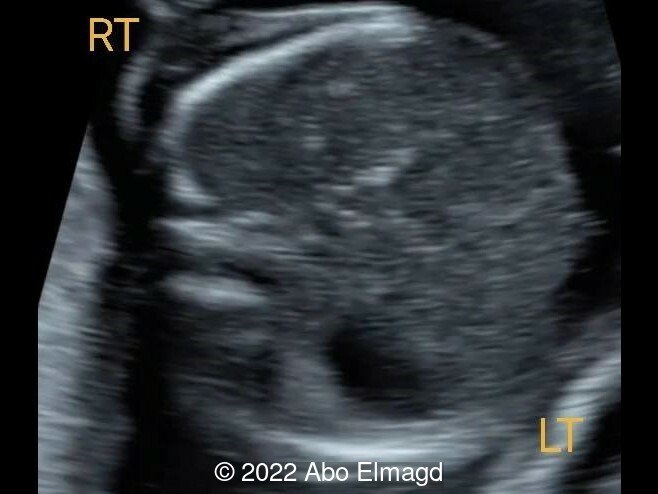
- Image 1: Image demonstrating the Situs
-
Image 2: Reduced filling of the left ventricle with stagnant blood flow due to poor contractility.
-
Image 3: Mitral valve regurgitation during systole.
-
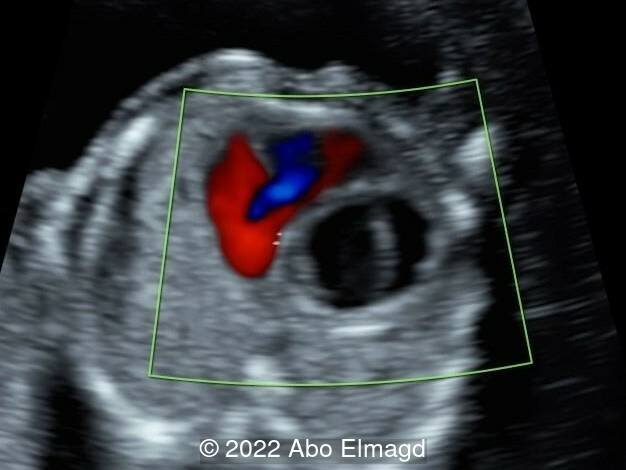
Image 4: Left-to-right shunting at the level of the foramen ovale.
-
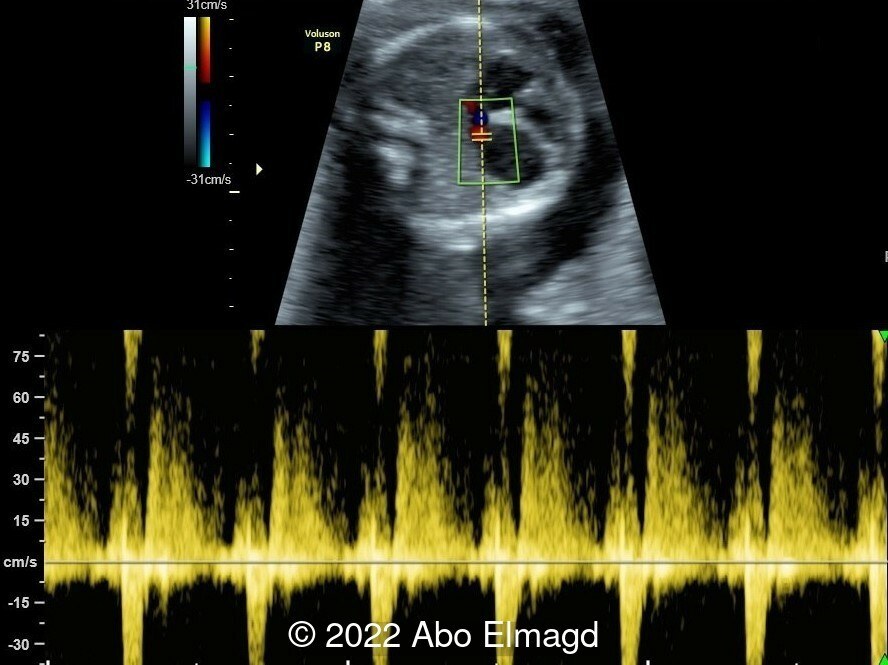
Video 3: Post stenotic dilation of the ascending aorta with turbulent flow due to narrow aortic root and reduced motion of valve leaflets.
-
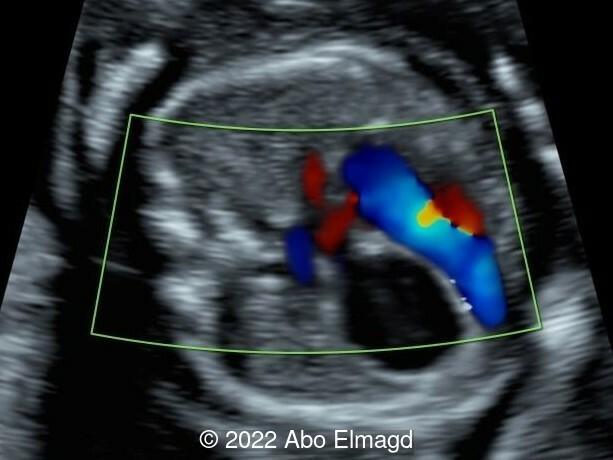
Image 5 and Video 4: Reversed flow in the aortic arch in late systole and diastole.
Discussion
Aortic stenosis is defined as narrowing of the patent aortic valve leading to left outflow tract obstruction [1]. There is secondary dysfunction of the left ventricular myocardium, impaired left ventricular contractility, stagnation of blood and deposition of fibrin along the ventricular wall leading to fibroelastosis [2]. The left ventricle may be dilated or hypoplastic, as in hypoplastic left heart syndrome. Aortic stenosis spectrum varies markedly from mild (normal ventricular size, contractility and myocardial echogenicity, with antegrade flow across the mitral valve) to critical aortic stenosis, as described here. Mild aortic stenosis may progress throughout gestation to critical aortic stenosis. [3]
Aortic stenosis represents 3-6% of structural heart defects [1]. Associated cardiac malformations are found in approximately 20% of patients and include ventricular septal defect, coarctation of the aorta and patent ductus arteriosus (postnatally). Extracardiac malformations are uncommon in aortic stenosis and association with chromosomal abnormalities is also rare [4].
Differential diagnoses include hypoplastic left heart syndrome from an alternative aetiology, isolated endocardial fibroelastosis, dilated left ventricular cardiomyopathy, and aorto-left ventricular tunnel. These conditions are relatively rare compared to critical aortic stenosis [5].
Follow up ultrasound evaluation of aortic stenosis is recommended every 2 to 4 weeks to assess for deterioration of the disease [6]. Decreasing contractility and increasing echogenicity of the left ventricular wall, as well as a decrease in the peak systolic aortic velocity, can be considered signs of progression and worsening prognosis [7,8]. Progression of aortic stenosis to hypoplastic left heart syndrome has been reported [2,5,7,8].
The prognosis for this condition is poor. Postnatal mortality rate is 50%, even with surgical intervention. If there is adequate ventricular function after birth, balloon valvuloplasty is preferred. If ventricular function is poor, univentricular palliation is performed [9].
In severe cases with high risk of fetal mortality, in utero aortic valvuloplasty has been performed. Modest outcomes have been reported even in hydropic fetuses, however case numbers are small, results are conflicting, and long term follow up data is lacking [9,10]. Certain parameters have been identified which may predict good outcomes and can be used to inform patient selection [11,12]. Further studies are in progress [13].
References
[1] Ferencz C, Rubin JD, Loffredo CA, et al. Epidemiology of congenital heart disease: the Baltimore-Washington infant study 1981–1989. Austin, TX: Futura Publishing, 1993;38.
[2] Hornberger LK, Sanders SP, Rein AJ, et al. Left heart obstructive lesions and left ventricular growth in the midtrimester fetus. A longitudinal study. Circulation 1995;92(6):1531–1538.
[3] Abuhamad, A et al. Aortic Stenosis and Biscuspid Aortic Valve. A Practical Guide to Fetal Echocardiography. Philadelphia: Wolters Kluwer, 2022. pgs 448- 460.
[4] Braunwald E, Goldblatt A, Aygen MM, et al. Congenital aortic stenosis. I. Clinical and hemodynamic findings in 100 patients. II. Surgical and the results of operation. Circulation 1963;27:462.
[5] Sharland GK, Chita SK, Fagg NL, et al. Left ventricular dysfunction in the fetus: relation to aortic valve anomalies and endocardial fibroelastosis. Br Heart J 1991;66(6):419–424.
[5] Sharland GK, Chita SK, Fagg NL, et al. Left ventricular dysfunction in the fetus: relation to aortic valve anomalies and endocardial fibroelastosis. Br Heart J 1991;66(6):419–424.
[6] Drury NE, Veldtman GR, Benson LN. Neonatal aortic stenosis. Expert Rev Cardiovasc Ther 2005;3(5):831–843.
[7] Allan LD, Sharland G, Tynan MJ. The natural history of the hypoplastic left heart syndrome. Int J Cardiol 1989;25(3):341–343.
[8] Simpson JM, Sharland GK. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart 1997;77(3):205–210.
[9] National Institute for Health and Care Excellence 2018. Percutaneous balloon valvuloplasty for fetal critical aortic stenosis. Interventional Procedures Guidance.
[10] Tulzer, A., Arzt, W. and Tulzer, G. (2021), Fetal aortic valvuloplasty may rescue fetuses with critical aortic stenosis and hydrops. Ultrasound Obstet Gynecol, 57: 119-125.
[11] Tworetzky W, Wilkins-Haug L, Jennings RW, et al. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation 2004;110(15):2125–2131.
[12] Makikallio K, McElhinney DB, Levine JC, et al. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for fetal intervention. Circulation 2006;113(11):1401–1405.
[13] U.S. National Library of Medicine. “Fetal Intervention for Aortic Stenosis and Evolving Hypoplastic Left Heart Syndrome.” https://clinicaltrials.ucsf.edu/trial/NCT01736956. Web. Accessed 12 September 2022.
Discussion Board
Winners

Dianna Heidinger United States Sonographer

Javier Cortejoso Spain Physician

Seadet Zeynalova Azerbaijan Physician

Pawel Swietlicki Poland Physician

Fatih ULUC Turkey Physician

Margarita Alvarez de la Rosa Rodríguez Spain Physician

Umber Agarwal United Kingdom Maternal Fetal Medicine

Igor Yarchuk United States Sonographer

belen garrido Spain Physician

Andrii Averianov Ukraine Physician

Ana Ferrero Spain Physician

Mayank Chowdhury India Physician
Omayyah Dar Odeh Jordan Physician

Oskar Sylwestrzak Poland Physician

Vladimir Lemaire United States Physician

Shilpen Gondalia India Physician

Ivan Ivanov Russian Federation Physician

Boujemaa Oueslati Tunisia Physician

Michal Michna Slovakia Physician

Halil Mesut Turkey Physician

Anita Silber Israel Physician

RANJAN DUTTA India Physician

lan nguyen xuan Viet Nam Physician

Crismaru Iulia Romania Physician

Suat İnce Turkey Physician

Amparo Gimeno Spain Physician

Ta Son Vo Viet Nam Physician

Murat Cagan Turkey Physician

Umutcan KAYIKÇI Turkey Physician

Siddhesh Rajiwade India Maternal fetal medicine specialist
Mária Brešťanská Slovakia Physician

GOYAL MANISH United States

Ionut Valcea Romania Physician

José Lambertino Colombia Physician

Halil Korkut Dağlar United States Physician
KHALED RAMADAN Egypt Physician

mahmoud elbohy Egypt Physician

Seda Cam Turkey Physician

liesbeth lewi Belgium Physician

Pradeep Kumar India Physician

Sumit Singhania India Radiologist

Dr Monika Sharma India Physician

Molly Siemens United States Sonographer

Jose Saldaña Peru Physician

Subapriya Kandasamy India Physician
JOSE DERBY MEJIA FERNANDEZ Peru Physician

Hanna Moczulska Poland Physician