Case of the Week #560
(1) Radiologist, Imagerie Capricorne, Reunion Island; (2) Obstetrician, Felix Guyon Hospital, Reunion Island; (3) Clinical geneticist, Felix Guyon Hospital, Reunion Island
Posting Dates: May 31-June 14, 2022
Case Report: A 23-year-old G5P4 woman from Mayotte with no past history presented at 22 weeks gestation. The Down syndrome screening was 1 in 900 and subsequent fetal cDNA exam was normal. The mother's serologies were positive for Rubeola, though negative for Toxoplasmosis and Syphilis. The male fetus was found to have intrauterine growth restriction and the following findings:
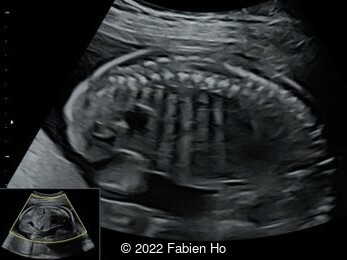
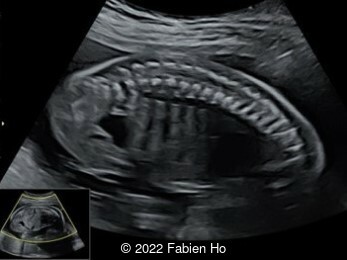
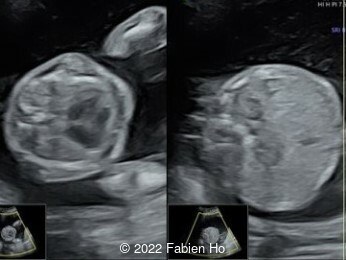
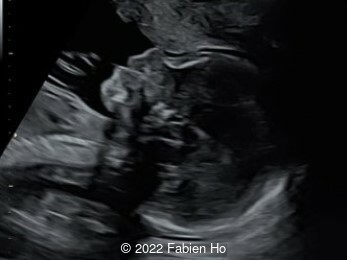
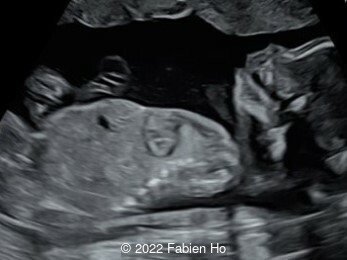
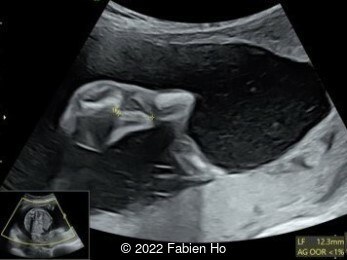
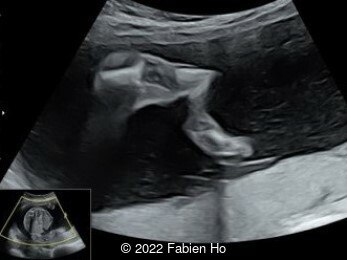
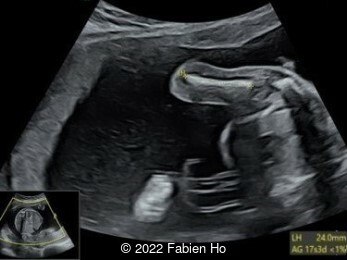
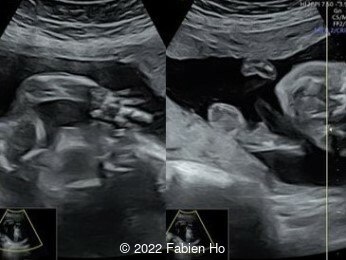
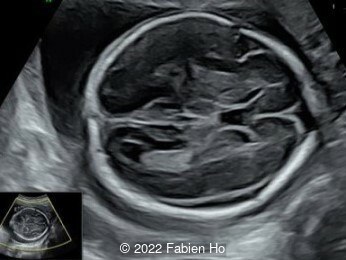
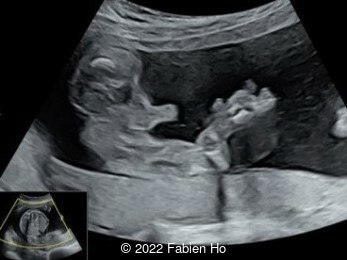
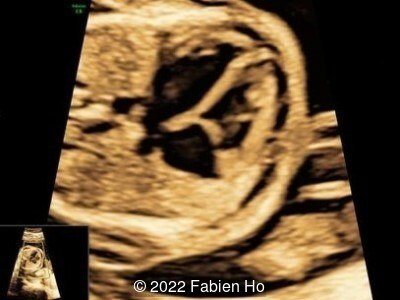
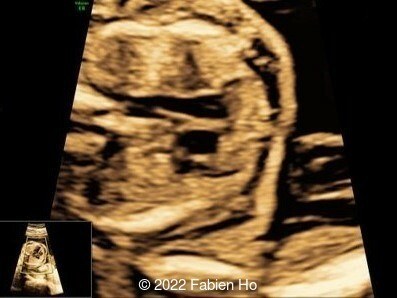
A CT scan was subsequently performed at 32 weeks gestation
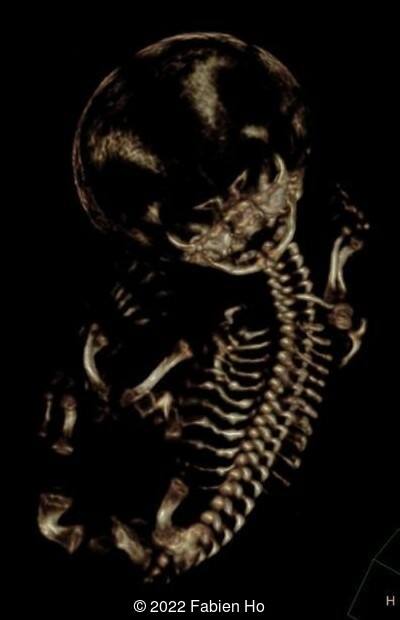
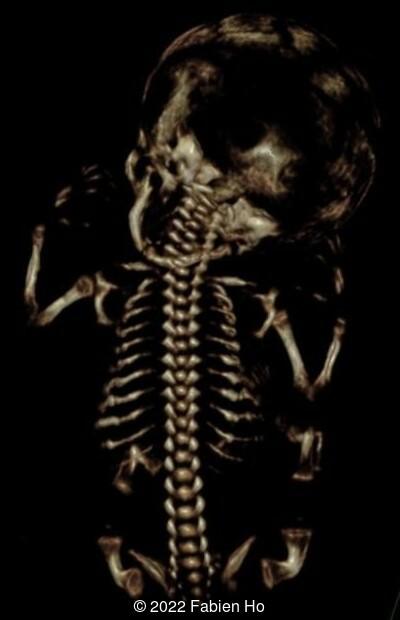
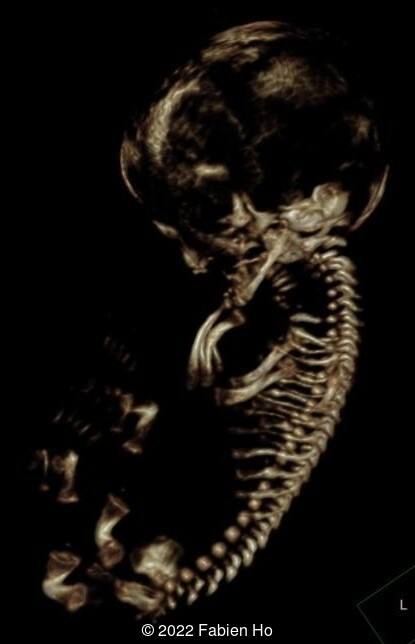
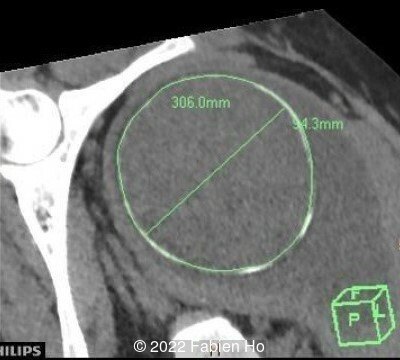
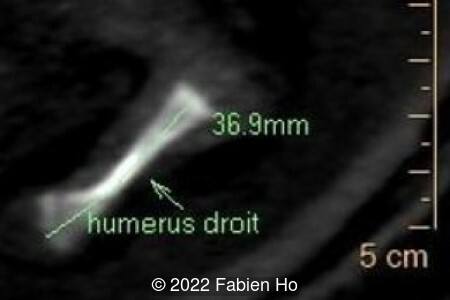
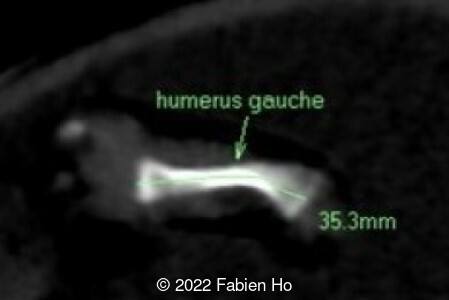
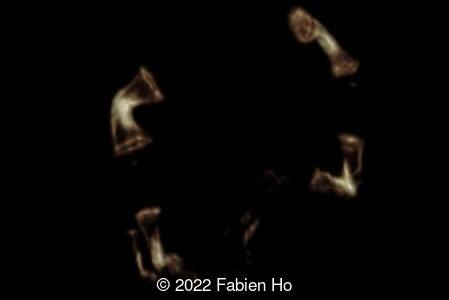
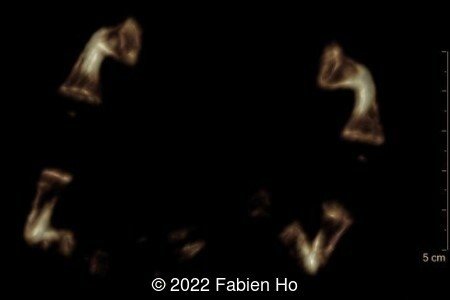
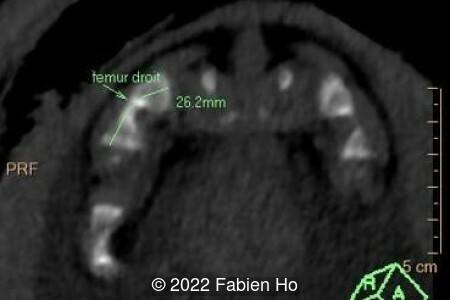
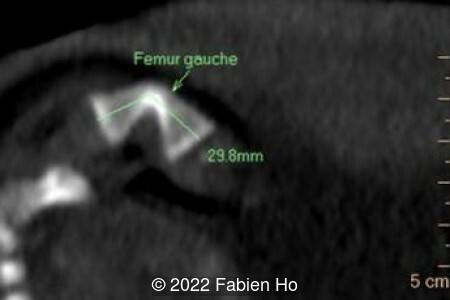

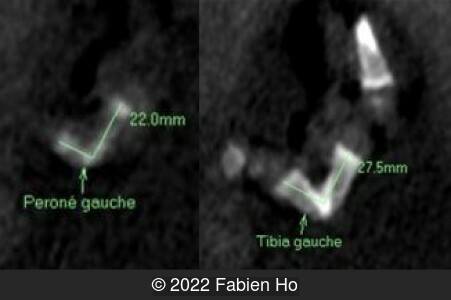
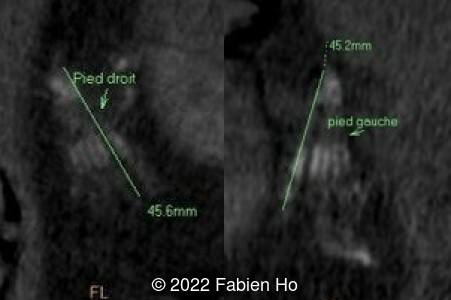
View the Answer Hide the Answer
Answer
We present a case of Osteogenesis Imperfecta.
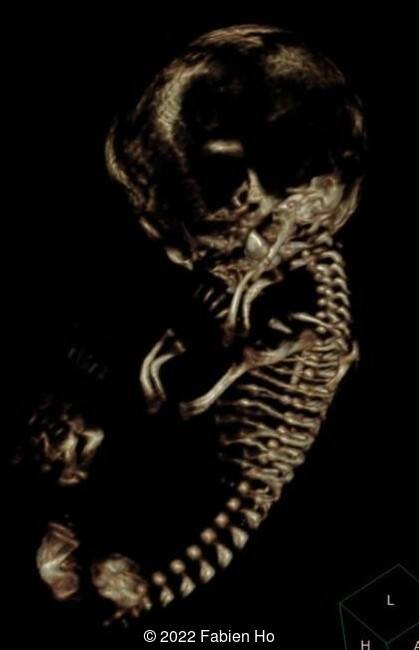
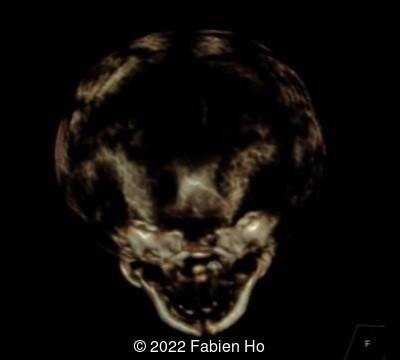
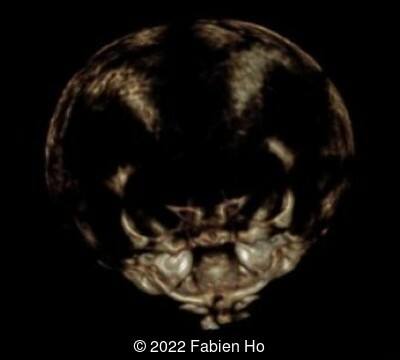
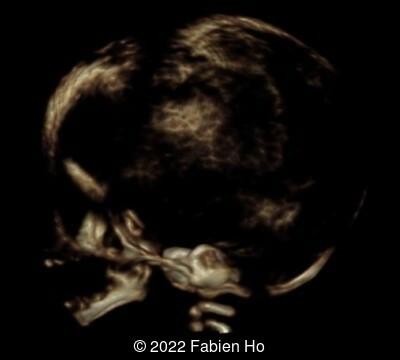
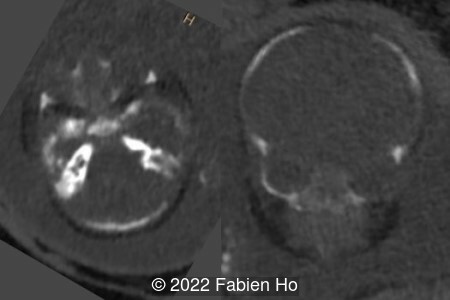
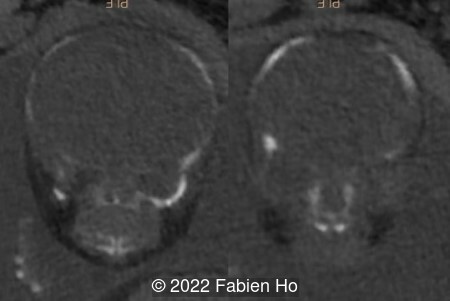
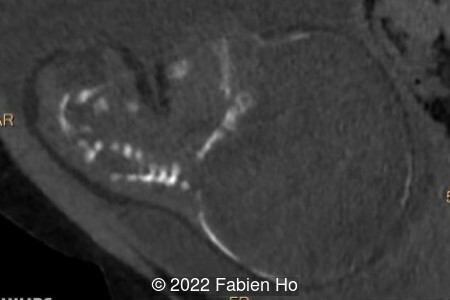
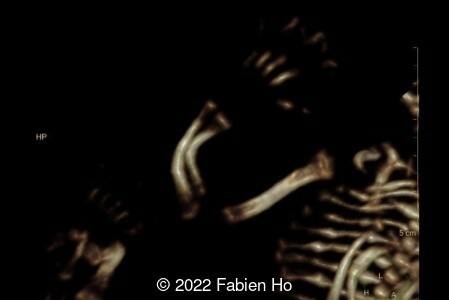
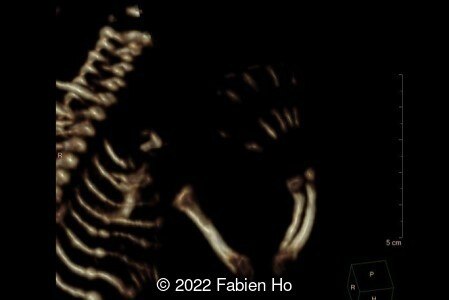
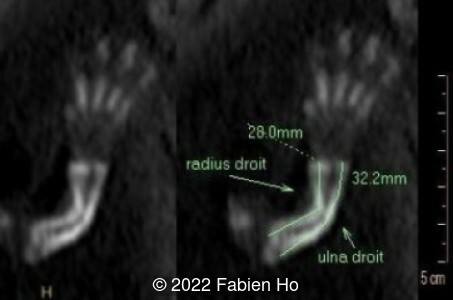
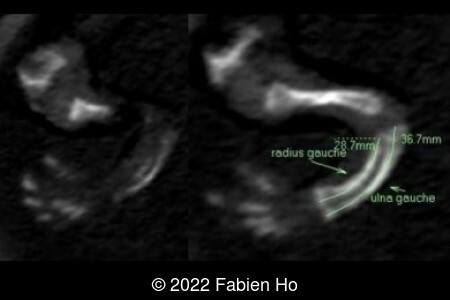
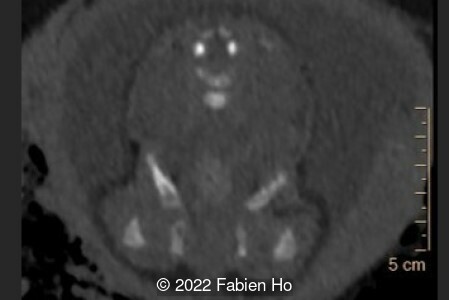
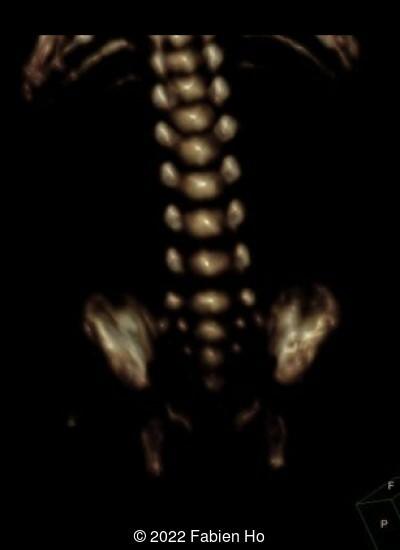
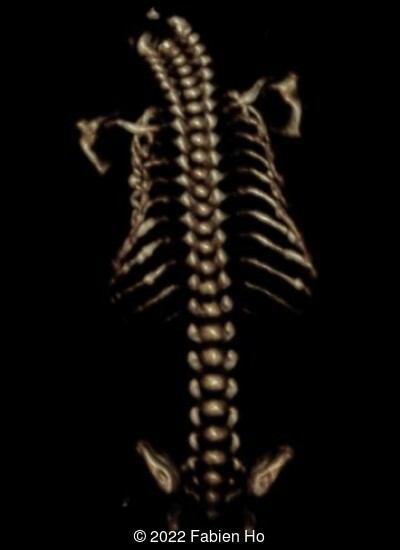
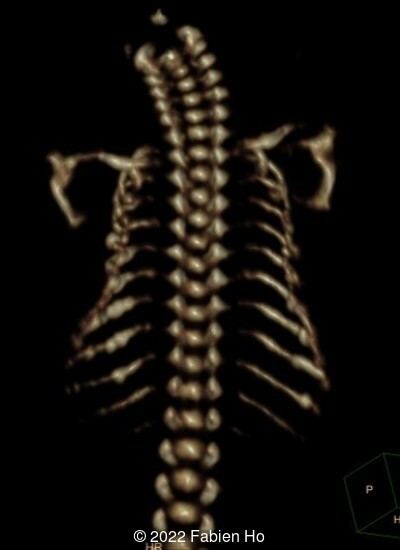
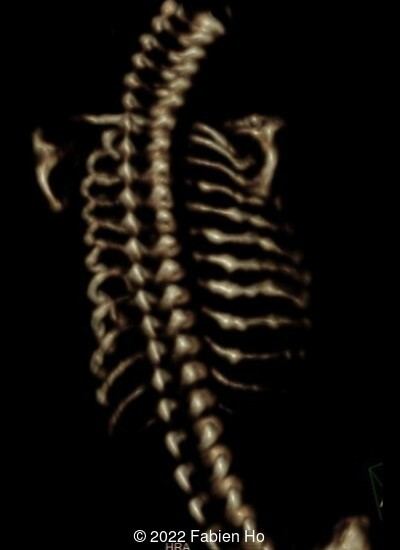
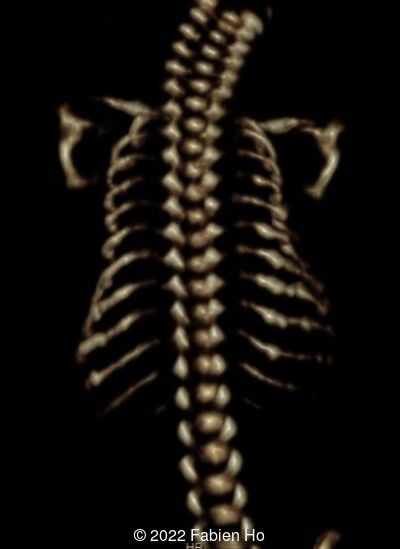
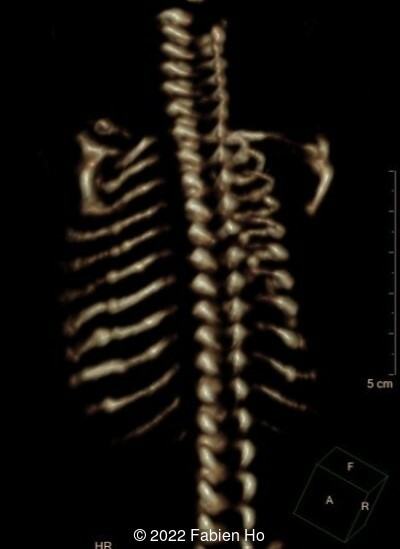
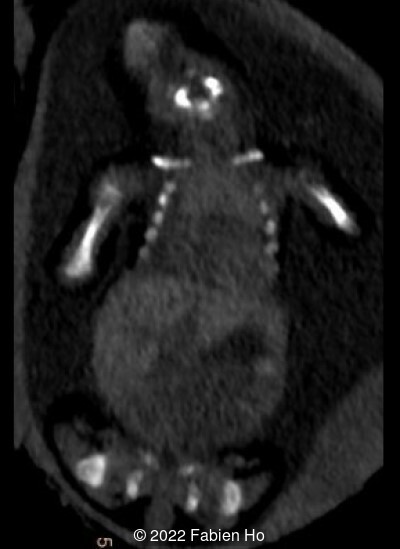
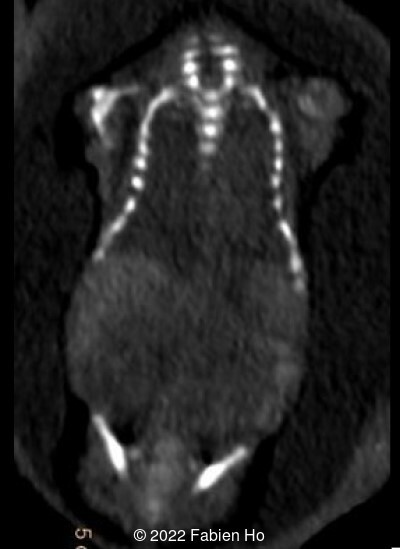
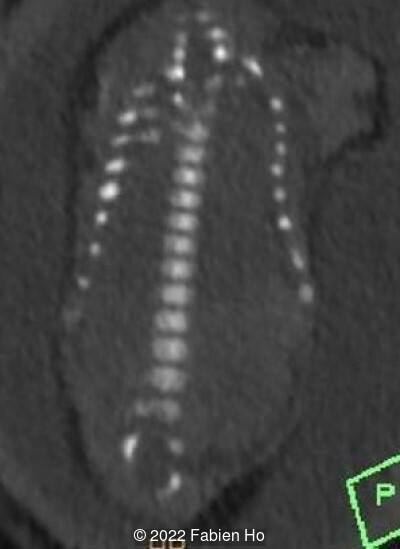
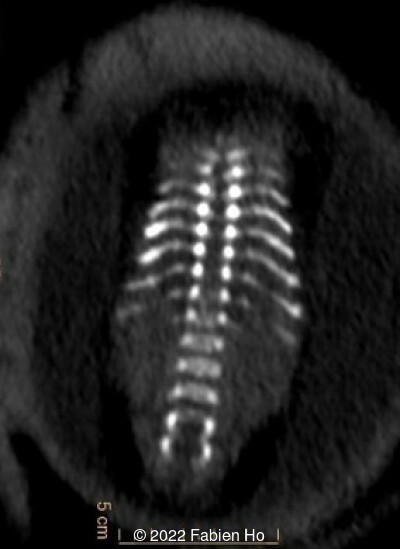
The prenatal CT scan showed:
- Low bone density of skull and lower limbs
- Moderately narrowed and bell-shaped thorax
- Numerous bone calluses of the posterior arcs of the ribs
- Normal bone density of the spine
- No platyspondyly
- Normal pelvis without iliac spine or other anomaly
- Very short long bones with curved femurs, tibias and fibulas. The curvature of the long bones reaches a 90° angle concerning for fracture. Upper limbs are moderately curved without fracture.
Counselling was offered and the parents chose to continue the pregnancy. A Caesarean section was performed at 38 weeks gestation with the birth of a 2520g male infant, APGARS of 4, 7, 9 at 1, 5, and 10 minutes. The infant initially had respiratory distress, though recovered spontaneously. On physical exam, the neonate was found to have obvious craniotabes (soft skull upon palpation), short thorax and short limbs. A postnatal x-ray showed platyspondyly, calluses on the ribs and left ulna, low bone density of the long bones, skull and spine, as well as marked curvature of the long bones. The infant has survived to 1 month of age at the writing of this report.
Osteogenesis imperfecta is a clinically and genetically heterogeneous disorder of collagen characterized by bone fragility and deformity due to repetitive fractures. It can be due to a defective production, secretion or function of type 1 collagen found in tendons, ligaments and bones [1]. The incidence is reported as 0.42 in 10,000 births [2]. In a more recent study, the prevalence of osteogenesis imperfecta in the Swedish pediatric population was calculated to be 0.74 in 10,000 [3].
Sillence et al proposed four categories of osteogenesis imperfecta in 1979 based on specific phenotypes [4]:
- Type 1 is the mildest and presents with blue sclera, brittle teeth (dentinogenesis imperfecta), hearing loss, cardiac valvular regurgitation but no growth restriction. It is related to a quantitative deficiency of structurally normal collagen.
- Type 2 is the most extreme form. It can be diagnosed prenatally and is known to be lethal.
- Type 3 is the most severe survivable form and presents with severe progressive deformities and short stature.
- Type 4 is of intermediate severity presenting with hypertrophic calluses and calcification of interosseus membranes [4].
Osteogenesis imperfecta can be autosomal dominant inheritance, most commonly a mutation in the COL1A1 and COL1A2 genes which encode the α1 and α2 chains of type 1 collagen. Collagen molecules are composed of three polypeptide chains, forming a triple helix. Glycine is essential for the formation of the triple helix and mutations causing glycine substitutions disrupt the helix [5]. Substitutions in the α1 chain results in lethal outcomes while substitutions in the α2 chains are usually nonlethal [5]. Most cases of osteogenesis imperfecta are caused by the autosomal dominant or de novo mutations, though rare autosomal recessive and X-linked mutations have also been identified [6]. In a study reviewing 37 parental pairs and 216 affected infants, the recurrence rate after the first affected pregnancy was 1.3% and the overall recurrence rate for couples after two or more affected infants was 32% [7].
Characteristics of osteogenesis imperfecta that can be identified prenatally include early onset of severe long bone shortening and bowing due to multiple intrauterine fractures, narrow thorax, normal head circumference but abnormal translucency and compressibility [8,9]. The poor mineralization of the skull leads to deformation of the head by the ultrasound transducer [10]. The diagnosis can be made as early as the first to early second trimester [11]. Several cases have also been documented in the literature that associate osteogenesis imperfecta with an increased nuchal translucency in the first trimester [12].
The differential diagnosis of osteogenesis imperfecta includes:
- Achondrogenesis which is characterized by extremely short limbs, small chest, macrocrania, and decreased mineralization with occasional fractures [1]. In our case, the limb micromelia is significant but not extreme. Additionally, the spine bone density is normal and there is no platyspondyly.
- Thanatophoric dysplasia is the most common lethal skeletal dysplasia and characterized by extremely short limbs, small chest, macrocrania, frontal bossing, cloverleaf skull, and normal mineralization without fractions [1]. In our case, the femurs are curved, however patients with thanatophoric dysplasia have platyspondyly, pelvic bone anomalies, and flared metaphysis of the long bones.
- Hypophosphatasia can also present with shortening of the limbs and low ossification of the skeleton [13].
- Ciliopathy such as short rib-polydactylia syndrome or Ellis-van Creveld syndrome. In our case, the bell-shaped thorax is not the dominant finding as it is in short rib-polydactylia and there are no visceral additional anomalies as seen in Ellis-van Creveld syndrome.
It can be difficult to distinguish between lethal (type 2) and severe (type 3) osteogenesis imperfecta in utero. Munoz et al proposed a triade for the diagnosis of lethality which includes marked femoral shortening (femur length >3 standard deviations below the mean for gestational age), multiple fractures in a single bone, and demineralization of the calvarium [8]. Biometric characteristics that have been used to predict lethality are femur length-to-abdominal circumference ratio (FL-to-AC) and fetal lung volume [1]. A FL-to-AC ratio of <0.16 is a reliable predictor of lethal skeletal dysplasia [14,15]. Hypoplastic lungs are defined as a lung volume <5th percentile expected for gestational age. In a study by Weaver et al, a ratio of observed to expected total lung volume of <47.9% had a sensitivity of 75% and specificity of 82% [16]. Additionally, fetuses with lethal skeletal dysplasia were more likely to have polyhydramnios [15].
Prenatal diagnosis is made by culturing the patients’ fibroblast obtained with chorionic villus sampling and analyzing the collagen products [1,11]. More recent techniques use cell-free fetal DNA in the maternal peripheral blood for genetic testing [17]. Due to the heterogeneity of genes causing osteogenesis imperfecta, cell-free fetal DNA testing may be inaccurate if the mutation is not within the dominant COL1A1 and COL1A2 genes [1].
In patients with osteogenesis imperfecta, death occurs due to respiratory infections, kyphoscoliosis leading to cardiac failure, and intracranial hemorrhage as a result of trauma [18]. Survival ranges from a few hours to several months in patients with type IIB [10]. Death in these patients usually arises from intracranial hemorrhage due to vessel fragility or respiratory distress due to pulmonary hypoplasia [10]. Previously, Cesarean delivery was assumed to be safer however recent studies show that delivery mode does not affect the rate of at-birth fractures [19].
References
[1] Deguchi M, Tsuji S, Katsura D, et al. Current Overview of Osteogenesis Imperfecta. Medicina (Kaunas). 2021 May 10;57(5):464.
[2] Orioli IM, Castilla EE, Barbosa-Neto JG. The birth prevalence rates for the skeletal dysplasias. J Med Genet. 1986 Aug;23(4):328-32
[3] Lindahl K, Åström E, Rubin CJ et al. Genetic epidemiology, prevalence, and genotype-phenotype correlations in the Swedish population with osteogenesis imperfecta. Eur J Hum Genet. 2015 Aug;23(8):1042-50.
[4] Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101-16.
[5] Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2016 Apr 16;387(10028):1657-71.
[6] Marini JC, Blissett AR. New genes in bone development: what's new in osteogenesis imperfecta. J Clin Endocrinol Metab. 2013 Aug;98(8):3095-103.
[7] Pyott SM, Pepin MG, Schwarze U. Recurrence of perinatal lethal osteogenesis imperfecta in sibships: parsing the risk between parental mosaicism for dominant mutations and autosomal recessive inheritance. Genet Med. 2011 Feb;13(2):125-30.
[8] Muñoz C, Filly RA, Golbus MS. Osteogenesis imperfecta type II: Prenatal sonographic diagnosis. Radiology. 1990 Jan;174(1):181-5.
[9] Schramm T, Gloning KP, Minderer S et al. Prenatal sonographic diagnosis of skeletal dysplasias. Ultrasound Obstet Gynecol. 2009 Aug;34(2):160-70.
[10] Berge LN, Marton V, Tranebjaerg L, et al. Prenatal diagnosis of osteogenesis imperfecta. Acta Obstet Gynecol Scand. 1995 Apr;74(4):321-3.
[11] DiMaio MS, Barth R, Koprivnikar KE et al. First-trimester prenatal diagnosis of osteogenesis imperfecta type II by DNA analysis and sonography. Prenat Diagn. 1993 Jul;13(7):589-96.
[12] Hsieh CTC, Yeh GP, Wu HH et al. Fetus with osteogenesis imperfecta presenting as increased nuchal translucency thickness in the first trimester. J Clin Ultrasound. 2008 Feb;36(2):119-22.
[13] Offiah AC, Vockley J, Munns CF et al. Differential diagnosis of perinatal hypophosphatasia: radiologic perspectives. Pediatr Radiol. 2019; 49(1): 3–22.
[14] Rahemtullah A, McGillivray B, Wilson RD. Suspected skeletal dysplasias: femur length to abdominal circumference ratio can be used in ultrasonographic prediction of fetal outcome. Am J Obstet Gynecol. 1997 Oct;177(4):864-9.
[15] Nelson DB, Dashe JS, McIntire DD et al. Fetal skeletal dysplasias: sonographic indices associated with adverse outcomes. J Ultrasound Med. 2014 Jun;33(6):1085-90.
[16] Weaver KN, Johnson J, Kline-Fath B, et al. Predictive value of fetal lung volume in prenatally diagnosed skeletal dysplasia. Prenat Diagn. 2014 Dec;34(13):1326-31.
[17] Dan S, Yuan Y Wang Y et al. Non-Invasive Prenatal Diagnosis of Lethal Skeletal Dysplasia by Targeted Capture Sequencing of Maternal Plasma. PLoS One. 2016 Jul 19;11(7):e0159355.
[18] McAllion SJ, Paterson CR. Causes of death in osteogenesis imperfecta. J Clin Pathol. 1996 Aug;49(8):627-30.
[19] Cubert R, Cheng EY, S Mack S, et al. Osteogenesis imperfecta: mode of delivery and neonatal outcome. Obstet Gynecol. 2001 Jan;97(1):66-9.
Discussion Board
Winners

Javier Cortejoso Spain Physician

Seadet Zeynalova Azerbaijan Physician

Fatih ULUC Turkey Physician

Igor Yarchuk United States Sonographer

Andrii Averianov Ukraine Physician

Ana Ferrero Spain Physician

Alexandr Krasnov Ukraine Physician

Carlos Orellana Venezuela Physician

Mayank Chowdhury India Physician
Omayyah Dar Odeh Jordan Physician

DAVID BEAUMONT United Kingdom Physician

Boujemaa Oueslati Tunisia Physician

Tatiana Koipish Belarus Physician

Halil Mesut Turkey Physician

Anita Silber Israel Physician

Nguyen Luân Viet Nam Physician

Suat İnce Turkey Physician

Victoria Giang Viet Nam Physician

Ta Son Vo Viet Nam Physician

Sonio Sonio France AI

Rasha Abo Almagd Egypt Physician

Inass Osman United Kingdom Physician

Ahmed Abujaber Canada Physician

Keya Malde Australia Sonographer
KHALED RAMADAN Egypt Physician

Viralkumar Madhu India Sonographer

Thi Thanh Tu Le Viet Nam Physician

Luis Goncalves United States Physician

nadia toma United States